Scientific Studies Database By Natural Foundation
We've collected over 1000 studies on the products we sell. We've compiled the most interesting studies on this page.
We stay on top of the latest scientific research to ensure our products are as effective and safe as possible.
No studies found! Try another keyword.
Blood Pressure Support Scientific Studies
Our Blood Pressure Support product is designed with key ingredients backed by scientific studies. It includes high-nitrate beetroot, potassium, and magnesium, which are known to support healthy blood pressure levels. Research indicates that these nutrients, in effective doses, can promote cardiovascular health safely and naturally. We have summarised the most interesting scientific studies.
Study 2
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
250 mL/day of beetroot juice (containing ~6.4mmol of nitrate) or a placebo (250 mL of nitrate-depleted beetroot juice)
Participants:
64 hypertensive men and women aged 18 to 85
Duration:
4 weeks
Results:
The researchers observed that daily supplementation with beetroot juice, which is high in dietary nitrate, significantly reduced blood pressure. The reduction in blood pressure was similar to what is typically seen with a standard dose of blood pressure medication. These significant results suggest that dietary nitrate from beetroot juice may be used as an additional therapy for managing high blood pressure..
Year:
2014
Link:
Study 3
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
314 mM of nitrate tablets or a placebo (nitrate-free tablets). The nitrate tablets consisted of nitrate-rich beetroot extract 20mg, thiamine mononitrate 90mg, potassium nitrate 480mg, ascorbic acid 150mg, folic acid 200mcg, methylcobalamin 200mcg, calcium 115mg, pomegranate fruit extract 5mg and green coffee bean extract 115mg. Ascorbic acid was added to facilitate NO bioavailability.
Participants:
67 hypertensive men and women with a mean age of 59
Duration:
12 weeks
Results:
The study found that nitrate tablets significantly reduced blood pressure, more so than a placebo. The upper number (systolic) dropped by 12.5 mmHg, while the lower number (diastolic) dropped by 4.7 mmHg. In contrast, the placebo group saw smaller or no significant changes. Additionally, the nitrate tablets improved endothelial function, which helps blood vessels work better. The researchers concluded that nitrate tablets effectively lower blood pressure and improve blood vessel health in people with high blood pressure.
Year:
2020
Link:
Study 4
Study type:
Randomised, single-blind, cross-over postprandial trial (pilot study)
Dose:
In study 1, participants were randomly assigned to consume either 0, 100, 250 or 500mL of beetroot juice (each having a nitrate concentration of <0.5, 2.3, 5.7 and 11.4 mmol respectively). In study 2, participants were randomly assigned to consume either 200g of unfortified bread (<0.5 mmol of nitrate) or 200g of bread fortified with white or red beetroot (1.6 and 1.8 mmol of nitrate respectively). All participants consumed a low-nitrate/nitrite diet for 1 day before each study.
Participants:
18 healthy normotensive males in study 1 and 14 healthy normotensive males in study 2.
Duration:
Acute: blood pressure was measured over a 24h period following the consumption of beetroot juice, beetroot-fortified bread and the controls.
Results:
Supplementation with beetroot juice or beetroot-fortified bread was associated with larger reductions in blood pressure than the control. The peak reduction in blood pressure occurred after 2-3 hours:
- 100mL of beetroot juice was associated with a 13.1/16.6 mmHg reduction in blood pressure.
- 250mL of beetroot juice was associated with a 20.5/14.6 mmHg reduction in blood pressure.
- 500mL of beetroot juice was associated with a 22.2/18.3 mmHg reduction in blood pressure.
- Bread fortified with white beetroot was associated with a 19.3/16.5 mmHg reduction in blood pressure.
- Bread fortified with red beetroot was associated with a 23.6/23.2 mmHg reduction in blood pressure.
These results suggest that nitrate in beetroot may significantly help to reduce blood pressure. The reductions in blood pressure after the consumption of fortified bread suggest that processed beetroot may lower blood pressure to a similar degree as unprocessed beetroot. Thus, beetroot supplements may help to reduce overall blood pressure.
Year:
2015
Link:
Study 5
Study type:
Randomised non-blinded postprandial trial
Dose:
The experimental group consumed 500mL of beetroot juice with a mean nitrate concentration of 45.0±2.6 mMol/L (2.79g/L). The control group consumed water.
Participants:
14 healthy subjects
Duration:
Acute: blood pressure was measured over a 24h period following the consumption of beetroot juice.
Results:
Beetroot juice supplementation (dietary nitrate) was associated with larger reductions in blood pressure than the control. Specifically, blood pressure dropped by 10.4/8 mmHg after 2.5 hours compared to the control. The drop in blood pressure was correlated with increases in plasma nitrite concentration. This suggests that dietary nitrate may underlie the beneficial effects of beetroot. After 24 hours, systolic blood pressure was 4.4 mmHg lower with beetroot juice than water.
Year:
2008
Link:
Study 6
Study type:
Randomised non-blinded crossover trial
Dose
250mL of beetroot juice (containing 5.5 mmol of nitrate) or placebo (250mL of water)
Participants:
9 healthy men and women.
Duration:
Acute (less than 24 hours)
Results:
Supplementation with beetroot juice (dietary nitrate) was associated with a 5.4 mmHg reduction in systolic blood pressure. The authors suggested that a dietary nitrate approach to cardiovascular disease may have therapeutic use.
Year:
2010
Link:
Study 7
Study type:
Randomised, double-blind, crossover trial
Dose
2 x 70 mL/day of organic beetroot juice (each containing 4.8 mmol of nitrate). The placebo consumed nitrate-depleted beetroot juice.
Participants:
12 healthy, normotensive, non-smoking, older adults (6 males and 6 female)
Duration:
2.5 days
Results:
2.5 days of dietary nitrate supplementation was associated with a four-fold increase in plasma nitrite concentration and significant reductions in resting blood pressure. More specifically, plasma nitrite increased to 418% of the placebo value, and blood pressure decreased by 5/3 mmHg relative to the placebo (115/70 vs 120/73 mmHg). The authors suggested that nitrate supplementation could potentially reduce the risk of hypertension and cardiovascular disease in older adults.
Year:
2013
Link:
Study 8
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Dose:
Nitrate-rich beetroot juice (12.9 mmol of nitrate) or a placebo (nitrate-depleted beetroot juice (0.5 mmol of nitrate)
Participants:
20 men and women (mean age: 62.5) with uncontrolled hypertension
Duration:
7 days
Results:
Supplementation with beetroot juice (dietary nitrate) was associated with a significant reduction in systolic and diastolic blood pressure compared to the placebo. Beetroot juice supplementation was also significantly associated with increased plasma nitrite. Significant decreases in 24h (−8/−4 mmHg) and day blood pressure (−9/−4 mmHg) profiles were observed.
Year:
2018
Link:
Study 9
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Dose:
150 mL of nitrate-rich beetroot juice (10.5 mmol of nitrate) or a placebo (1 mmol nitrate) 2.25 hours prior to a 30-min treadmill walk
Participants:
13 younger (18–30) and 11 older (50–70) normotensive adults
Duration:
Acute (3.5 hours)
Results:
Supplementation with beetroot juice (dietary nitrate) was associated with a significant reduction in systolic blood pressure in both age groups and diastolic blood pressure in older adults. Beetroot juice was also associated with increased plasma nitrate and nitrite concentrations.
The authors concluded that acute supplementation with beetroot may reduce blood pressure.
Year:
2019
Link:
Study 10
Study type:
Randomised, placebo-controlled, single-blind crossover trial
Dose:
140 mL of beetroot juice (containing 7.58 millimoles of nitrate) or a placebo (163 ml of prune juice with less than 0.01 millimoles of nitrate)
Participants:
15 patients with chronic obstructive pulmonary disease (11 males and 3 females)
Duration:
48 hours
Results:
Supplementation with beetroot juice (dietary nitrate) was associated with a significant reduction in resting systolic blood pressure (-8 mmHg), end-of-exercise diastolic blood pressure (-5 mmHg) and a trend for a decrease in resting diastolic blood pressure (−3 mmHg). Beetroot juice was also associated with increased plasma nitrate (+938%) and nitrite (+379%) relative to the placebo.
The authors concluded that dietary nitrate supplementation may reduce blood pressure and elevate plasma nitrate and nitrite concentrations.
Year:
2015
Link:
Study 11
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Dose:
Nitrate-rich beetroot juice (500mg/8.1mmol of nitrate) or a placebo (nitrate-depleted beetroot juice with less than 0.08 mmol of nitrate)
Participants:
18 untreated hypertensives aged 44 on average.
Duration:
Acute (8 hours)
Results:
Supplementation with beetroot juice (dietary nitrate) was associated with a larger reduction in ambulatory systolic and diastolic blood pressure (-6.7/-5.2 mmHg) compared to the placebo (-0.8/-1.7 mmHg) after 8 hours.
Year:
2018
Link:
Study 12
Study type:
Randomised controlled trial
Dose:
∼5.76 mmol of nitrate in the form of a concentrated beetroot juice drink (55 mL), a non-concentrated beetroot juice drink (456 mL) and a solid beetroot flapjack (60 g). A drink containing soluble beetroot crystals (∼1.40 mmol of nitrate) and a control drink (70mL of deionised water) were also ingested.
Participants:
10 healthy males
Duration:
Acute (24 hours)
Results:
Beetroot juice (dietary nitrate) was associated with lower blood pressure and higher concentrations of nitric oxide metabolites. All nitrate-rich vehicles in the study were associated with elevated plasma, salivary and urinary nitric oxide metabolites compared with baseline and the control.
Year:
2018
Link:
Study 13
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Dose:
70mL of beetroot juice (400 mg of nitrate) or a placebo (nitrate-depleted beetroot juice)
Participants:
14 healthy males (aged 22 on average)
Duration:
15 days
Results:
Compared with the placebo, beetroot juice (dietary nitrate) was associated with reductions in systolic and diastolic blood pressure, mean arterial pressure and total peripheral resistance at rest and during exercise.
Beetroot juice was also associated with significant increases in baseline concentrations of plasma nitrate and nitrite compared to the placebo.
Year:
2015
Link:
Study 14
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
500 mL/day of beetroot juice (containing ∼6.2 mmol of nitrate) or a placebo (500 mL/day of nitrate-depleted beetroot juice)
Participants:
9 normotensive, physically active males
Duration:
6 days
Results:
Short-term supplementation with beetroot juice (dietary nitrate) was associated with significant increases in plasma nitrite (+105%) and reductions in systolic blood pressure (-5 mmHg) in normotensive young men consuming a normal, balanced diet. The placebo had no effect on systolic, diastolic or mean arterial blood pressure.
Year:
2011
Link:
Study 15
Study type:
Randomised double-blind crossover trial
Dose
24 mmol of potassium nitrate (1488mg of nitrate) in capsules or a placebo (24 mmol of potassium chloride)
Participants:
21 healthy men and women
Duration:
Acute (less than 24 hours)
Results:
Potassium nitrate supplementation was associated with substantial reductions in systolic and diastolic blood pressure over 24 hours, whereas a similar dose of potassium chloride did not alter blood pressure over the same time period. These findings suggest that the changes in blood pressure were not attributable to the potassium content. Instead, the changes were likely dependent on the endogenous conversion to nitrite and, thereupon, to nitric oxide: the changes in plasma nitrite correlated closely with reductions in blood pressure.
Kapil et al.’s findings also showed dose-dependent reductions in systolic blood pressure with incremental doses of inorganic nitrate (4mmol and 12mmol).
Year:
2010
Link:
Study 16
Study type:
Systematic review
Intervention under study:
Beetroot juice (dietary inorganic nitrate) supplementation
Studies reviewed:
11 randomised controlled trials published between 2008 and 2018
Results:
The review concluded that supplementation with beetroot juice may reduce blood pressure in different populations, probably through the nitrate-nitrite/nitric oxide pathway and secondary metabolites found in beetroot. The review also concluded that beetroot juice may significantly decrease the risk of suffering cardiovascular events, and the authors believe that beetroot juice should be promoted as a key component of a healthy lifestyle to control blood pressure in healthy and hypertensive individuals.
Year:
2018
Link:
Study 17
Study type:
Systematic review and meta-analysis
Intervention under study:
Dietary nitrate supplementation
Outcome under study:
Medium-term effects of dietary nitrate supplementation on systolic and diastolic blood pressure.
Studies included:
13 randomised clinical trials with 7 to 65 participants per study. Most of the trials were placebo-controlled (75%).
Results:
Overall, dietary nitrate supplementation for more than one week was associated with a significant decrease in systolic and diastolic blood pressure. The pooled effect for the two interventions showed a reduction in systolic blood pressure (-4.1 mmHg) and diastolic blood pressure (-2.0 mmHg).
Year:
2017
Link:
Study 18
Study type:
Systematic review and meta-analysis
Intervention under study:
Dietary nitrate supplementation. The intervention time ranged from 3 to 60 days with daily dosages of 70–250 mL of beetroot juice.
Outcome under study:
The role of dietary nitrate in lowering blood pressure in patients older than 18 with arterial hypertension ( > 130/80 mmHg).
Studies included:
7 single/double-blinded randomised controlled trials published between 2013 and 2020.
Results:
Inorganic nitrate derived from beetroot juice was associated with reductions in systolic blood pressure in patients with arterial hypertension. The authors concluded that dietary nitrate from beetroot juice may be an effective method to reduce the blood pressure of patients with arterial hypertension (in interventions up to 2 months duration).
Year:
2022
Link:
Study 19
Study type:
Systematic review and meta-analysis
Intervention under study:
Dietary inorganic nitrate/nitrite
Outcome under study:
Prevention and treatment of cardiovascular disease risk factors in humans
Studies included:
34 studies were included for qualitative synthesis, 23 of which were eligible for meta-analysis.
Results:
Inorganic nitrate intake was associated with significant reductions in resting blood pressure (systolic blood pressure: -4.80 mmHg; diastolic blood pressure: 1.74 mmHg).
Year:
2018
Link:
Study 20
Study type:
Randomised, double-blind, placebo-controlled trial
Dose
Beetroot juice with 6.5–7.3 mmol of nitrate. The placebo group received nitrate-depleted beetroot juice(<0.06 mmol nitrate)
Participants:
15 healthy, normotensive men and women aged 22 to 40
Duration:
Acute: 24 hours on two occasions (crossover)
Results:
Supplementation with beetroot juice containing inorganic nitrate was associated with lower aortic systolic blood pressure after 30 minutes (-5.2 mmHg).
Year:
2019
Link:
Study 21
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Dose:
70mL of beetroot juice (6.1 mmol nitrate). The placebo group consumed 70mL of nitrate-depleted beetroot juice.
Participants:
20 patients suffering from heart failure with preserved ejection fraction (aged 69 on average)
Duration:
First phase: acute (24 hours)
Second phase: 1 week
Results:
In both study phases, supplementation with nitrate-rich beetroot juice was associated with significant reductions in systolic blood pressure (resting) and increases in plasma nitrate and nitrite. After a single, acute dose of nitrate-rich beetroot juice, resting systolic blood pressure was significantly lower than the placebo (127 mmHg vs. 134 mmHg).
Compared to the placebo, plasma nitrite levels increased significantly after the nitrate-rich beetroot juice (38% after an acute dose and 129% after 1 week of daily doses).
The authors concluded that beetroot may significantly improve blood pressure in elderly patients with heart failure.
Year:
2016
Link:
Study 22
Study type:
Randomised placebo-controlled crossover trial
Dose
500 mL/day of beetroot juice (5.2 mmol of nitrate/day) and placebo (500 mL/day of juice). Participants also engaged in moderate-intensity exercise after 2.5 hours and on day 5 and day 15.
Participants:
8 healthy subjects (5 males and 3 females) with an average age of 29
Duration:
Acute (2.5 hours) and chronic (up to 15 days)
Results:
Nitrate-rich beetroot juice supplementation was associated with significantly lower systolic and diastolic blood pressure throughout the supplementation period (∼-4%). It was also associated with significantly elevated plasma nitrite concentration (+39% after 2.5 hours; +25% after 5 days; and +46% after 15 days).
The results indicate that dietary NO3− supplementation may acutely reduce blood pressure, and that this effect may be maintained for at least 15 days if supplementation is continued.
Year:
2010
Link:
Study 23
Study type:
Randomised unblinded crossover trial
Dose:
Participants were assigned either:
1. 200mL of beetroot juice (with ~800mg of nitrate) and 40 minutes of moderate-intensity aerobic exercise
2. 200mL of low-nitrate fruit soda and the same exercise
3. 200mL of water (insignificant nitrate) and no exercise.
Participants:
14 non-hypertensive obese males
Duration:
Acute (24 hours)
Results:
Compared to the control, supplementation with beetroot juice (dietary nitrate) was associated with a reduction in systolic ambulatory blood pressure (-5.3 mmHg) up to 6 hours after ingestion and moderate-intensity aerobic exercise. Beetroot juice supplementation was also associated with a significantly higher plasma nitric oxide concentration up to 1 hour after ingestion.
The authors concluded that inorganic nitrate may have important therapeutic applications to decrease the blood pressure response to exercise when individuals have an increased cardiovascular risk.
Year:
2019
Link:
Study 24
Study type:
Uncontrolled clinical trial
Intervention:
Beetroot juice concentrate and blackcurrant juice
Participants:
21 men and women
Duration:
3 weeks
Results:
Beetroot juice concentrate was associated with a ~7.3mmHg reduction in daily systolic blood pressure after 3 weeks. However, beetroot juice supplementation was not associated with significant changes in resting clinic blood pressure or 24h ambulatory blood pressure.
Year:
2016
Link:
Study 25
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Dose:
500 mL/day of either nitrate-rich beetroot juice (containing 5.1 mmol of nitrate/day) or placebo (a drink with negligible nitrate content
Participants:
7 men aged 19 to 38
Duration:
6 days
Results:
Overall, systolic and diastolic was significantly lower after 6 days of nitrate supplementation (-7/7 mmHg). Relative to the placebo, nitrate supplementation was associated with reductions in systolic blood pressure (-5 mmHg), diastolic blood pressure (-2 mmHg) and mean arterial pressure (-2 mmHg).
Year:
2010
Link:
Study 26
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Dose:
0.11 mmol of nitrate per kg of body weight (a body mass-normalised moderate dose of nitrate) via beetroot juice.
Participants:
11 patients with peripheral artery disease
Duration:
Acute (∼1 hour)
Results:
Compared to the placebo, dietary nitrate supplementation was associated with reductions in peripheral and central systolic blood pressure (−4.7 mmHg and −8.2 mmHg, respectively) and significant increases in serum nitrate/nitrite level.
The authors concuded that acute, body mass-normalised, moderate doses of dietary nitrate may improve blood pressure and nitric oxide bioavailability.
Year:
2021
Link:
Study 27
Study type:
Randomised crossover trial
Dose:
∼400mg of nitrate at lunch, provided through nitrate-rich vegetables or beetroot juice supplementation
Participants:
15 healthy men and women (aged 24 on average)
Duration:
1 week
Results:
Nitrate-rich vegetables and beetroot juice supplementation were associated with increases in plasma nitrate concentrations and reductions in mean systolic and diastolic blood pressure throughout both intervention periods (∼2.5 hours after lunch).
Year:
2020
Link:
Study 28
Study type:
Systematic review and meta-analysis
Intervention under study:
Dietary inorganic nitrate (with doses ranging from 150 to 1000mg over a treatment range from 7 to 168 days).
Outcome under study:
Effect of repeated administrations of inorganic nitrate on blood pressure and arterial stiffness.
Studies included:
47 randomised controlled trials, including 1101 participants (including healthy, overweight, hypertensive, diabetic and hypercholesterolemic individuals, and patients with heart failure and peripheral artery disease).
Results:
The results found that inorganic nitrate supplementation was associated with an overall beneficial effect on blood pressure. Repeated (≥ 3 days) administrations of inorganic nitrates were associated with reductions in peripheral and central blood pressure.
Year:
2020
Link:
Study 29
Study type:
Open-label randomised controlled trial
Dose:
70mL of beetroot concentrate (containing ~300 to 400mg of nitrate)
Participants:
21 overweight men and women between the ages of 55 and 70
Duration:
28 days
Results:
Supplementation with beetroot juice (dietary nitrate) was associated with a 10.2/3.1 mmHg reduction in blood pressure after 3 weeks of supplementation.
Year:
2015
Link:
Study 1
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To investigate the effect of potassium citrate or potassium chloride on blood pressure in volunteers with normal blood pressure.
Dose:
30 mmol/day of potassium citrate or potassium chloride or placebo
Participants:
127 men and women with an average age of 36 years
Duration:
6 weeks
Results:
The researchers observed significantly lowered blood pressure after taking potassium citrate for 6 weeks by about 5.22 mmHg. This reduction was similar to the 4.70 mmHg drop seen with potassium chloride. In addition, both forms of potassium reduced the upper and lower numbers of blood pressure, showing that potassium may help manage blood pressure levels.
Year:
2008
Link:
Study 2
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To investigate the long-term supplementation of potassium intake on blood pressure.
Dose:
7156.8 mg/day of potassium or placebo
Participants:
17 patients with essential hypertension (high blood pressure without any identifiable cause) with an average age of 29 years
Duration:
8 weeks
Results:
The study found an association between long-term potassium supplementation and significantly lowered blood pressure without changing salt intake. The average upper blood pressure value (systolic) significantly dropped from 152.2 mmHg to 137.4 mmHg, and the lower value (diastolic) significantly decreased from 99.6 mmHg to 89.1 mmHg. This effect was stronger in people with higher starting blood pressure and those who excreted more potassium. Lowering both systolic and diastolic blood pressure is important because it reduces the strain on your heart and arteries, lowering the risk of heart attacks, strokes, and other heart problems.
Year:
1985
Link:
Study 3
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To investigate the effects of short-term potassium supplementation on blood pressure in women with normal blood pressure
Dose:
3,128 mg/day of potassium or placebo
Participants:
39 healthy women with an average age of 32 years
Duration:
4 days
Results:
The researchers observed that potassium supplementation for 4 days significantly lowered systolic blood pressure by about 2 mmHg in normal blood pressure.
Year:
1991
Link:
Study 4
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To investigate the effect of long term oral potassium supplement in patients with mild hypertension.
Dose:
1876.8 mg/day of potassium supplements or placebo
Participants:
37 patients who had mildly increased blood pressure with an average age of 45 years
Duration:
15 weeks
Results:
By the third week, the researchers observed a significant decrease in blood pressure in the potassium group compared to the placebo group, with the maximum decrease occurring after 15 weeks. A subgroup of 13 patients who continued for an additional nine weeks with oral potassium supplements at half the previous daily dosage showed a moderate increase in blood pressure during this period compared to the values at the end of the full-dose treatment. At the end of this second study period, their blood pressure was still significantly lower than the values at the start of the study but not compared to the placebo group.
Year:
1987
Link:
Study 5
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To investigate the effects of potassium chloride on blood pressure in older adults with hypertension
Dose:
4692 mg/day of potassium chloride or placebo
Additional interventions:
A balanced diet that maintains the same calorie count daily (isocaloric diet) containing 4598 mg/day of sodium, 2737 mg/day of potassium, and 500 mg/day of calcium
Participants:
22 patients aged 60 years and older with mild to moderate essential hypertension
Duration:
8 days
Results:
During the potassium chloride treatment, the researchers observed that the participants’ average systolic blood pressure (upper value of BP reading) significantly dropped by 8.6 mmHg and diastolic blood pressure (lower value of BP reading) significantly dropped by 4.0 mmHg. There was no significant change in blood pressure with the placebo. Lowering both systolic and diastolic blood pressure reduces heart strain, decreases the risk of heart disease, and improves overall cardiovascular health.
Year:
1992
Link:
Study 6
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To evaluate the effect of a lower dose of potassium aspartate salt on blood pressure in individuals with essential arterial hypertension, a condition where a person has high blood pressure in their arteries without any specific identifiable cause.
Dose:
1173 mg/day of potassium aspartate or placebo
Participants:
104 male and female patients with an average age of 53 years
Duration:
4 weeks
Results:
The researchers observed that the control group showed no change in blood pressure, while the potassium supplementation group demonstrated significant reductions. For the potassium group, the average office blood pressure (measured at the doctor's office) significantly dropped from 154.4 mmHg to 142.2 mmHg, and the average 24-hour blood pressure (measured continuously over 24 hours using a portable monitor) significantly decreased from 142.7 mmHg to 134.8 mmHg. Overall, a relatively low dose of 1173 mg/day of potassium as aspartate may help lower blood pressure in people with mild to moderate high blood pressure.
Year:
2021
Link:
Study 7
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To explore the effects of potassium supplementation on blood pressure in hypertensive individuals.
Dose:
60 mmol of potassium or 40 g of soybean protein
Participants:
150 hypertensive men and women with an average age 56 years
Duration:
12 weeks
Results:
The researchers observed that after 12 weeks of potassium supplementation, those who took potassium supplements demonstrated a significant drop in their systolic blood pressure (upper number of the blood pressure reading) and a decreasing trend in their diastolic blood pressure (lower number of the blood pressure reading). This could suggest that the supplement may be particularly effective in reducing the strain on the heart during active pumping.
Year:
2001
Link:
Study 8
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To explore the effects of low-dose potassium supplementation on blood pressure in healthy volunteers.
Dose:
24 mmol/d of potassium or placebo
Participants:
59 male and female volunteers with an average age of 43 years
Duration:
6 weeks
Results:
The researchers observed that taking a low-dose potassium supplement daily for six weeks significantly lowered overall, systolic, and diastolic blood pressure in healthy volunteers. The greatest reduction was observed in those with a higher sodium-to-potassium ratio in their urine. This is beneficial because potassium helps balance the negative effects of sodium (salt) in the body. Studies have shown that consuming salt is often linked to high blood pressure, especially in salt-sensitive individuals. Overall, the findings suggest that taking potassium supplementation even at a low dosage may help improve blood pressure and overall heart health.
Year:
2003
Link:
Study 9
Study type:
Randomised crossover trial
Purpose:
To investigate the effects of potassium supplementation in patients with hypertension
Dose:
64 mmol/day of potassium
Participants:
55 patients with essential hypertension
Duration:
4 weeks
Results:
The study found an association between taking potassium supplements and significantly reduced blood pressure in people with high blood pressure. This reduction was observed in blood pressure readings taken at the doctor's office, at home, and over a full day while on potassium supplements compared to when they weren't taking the supplements.
Year:
1998
Link:
Study 10
Study type:
Clinical trial (Uncontrolled)
Purpose:
To investigate the effects of salt loading and potassium supplementation in individuals with normal blood pressure or mild hypertension.
Dose:
60 mmol /day of potassium
Additional interventions:
Low- and high-salt diet
Participants:
155 men and women with either normal blood pressure or mild hypertension.
Duration:
Around 4 weeks (1 week potassium supplementation at the final week)
Results:
The researchers observed that potassium supplements significantly reduced blood pressure and levels of endothelin-1 (a protein that tightens blood vessels) more in salt-sensitive people than in those who are not salt-sensitive. Salt-sensitive individuals have a greater increase in blood pressure when consuming salt, so dietary changes affect them more. Overall, the study highlights that potassium supplements can provide significant health benefits, especially for those sensitive to salt, who are more likely to have high blood pressure.
Year:
2013
Link:
Study 1
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To investigate the effects of magnesium supplementation on blood pressure in diabetic hypertensive adults with low magnesium levels.
Dose:
450 mg/day of magnesium, administered over a 4-month period.
Participants:
79 male and female diabetic hypertensive patients with an average age of 60 years old
Duration:
4 months
Results:
The study found an association between magnesium supplementation and significantly reduced blood pressure in diabetic hypertensive adults with low blood magnesium levels, compared to placebo. Additionally, the researchers also observed significantly increased HDL (good) cholesterol levels following magnesium supplementation. The study also found that people with lower magnesium levels in their blood are 2.8 times more likely to have high blood pressure compared to those with normal magnesium levels. Overall, magnesium may be a useful treatment for lowering blood pressure, especially in people with low magnesium levels.
Year:
2009
Link:
Study 2
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To investigate the effect of oral magnesium supplementation on blood pressure, in clinically healthy volunteers, including those with slightly high blood pressure or mildly high cholesterol.
Dose:
411-548 mg/day of magnesium or placebo
Participants:
33 male and female subjects with normal blood pressure or have borderline hypertension
Duration:
4 weeks
Results:
The group that took magnesium saw a significant decrease in their blood pressure, while the group that took a placebo did not. A decrease in both systolic (upper value of BP reading) and diastolic (lower value of BP reading) blood pressure means that the overall pressure in your blood vessels is lower, which is generally beneficial for heart health.
Year:
1997
Link:
Study 3
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To investigate the effect of oral magnesium supplementation on blood pressure in patients with mild hypertension
Dose:
1200 mg/day of magnesium (2 x 600 mg) or placebo
Participants:
28 mild hypertensive adults with an average age of 46 years
Duration:
12 weeks
Results:
The study found an association between oral magnesium supplementation and a significant reduction in 24-hour blood pressure. The researchers also observed that magnesium levels in the blood significantly increased in those taking the supplements, while no significant change in the control group.
Year:
2009
Link:
Study 4
Study type:
Single-arm non-blinded intervention study
Purpose:
To examine the effects of oral magnesium supplementation on blood pressure in patients with essential hypertension, a condition where a person has high blood pressure in their arteries without any specific identifiable cause.
Dose:
300 mg/day of oral magnesium-oxide supplementation
Participants:
48 men and women with essential hypertension
Duration:
1 month
Results:
The study found an association between magnesium supplements and significant decrease in blood pressure. On average, systolic pressure significantly dropped from 139.7 to 130.8 mmHg, and diastolic pressure significantly decreased from 88.0 to 82.2 mmHg.
Year:
2018
Link:
Study 5
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To observe the effects of oral magnesium-potassium supplementation on arterial compliance in essential hypertension. Arterial compliance refers to the ability of arteries to expand and contract with blood flow. High compliance means flexibility, while low compliance indicates stiffness, which can lead to high blood pressure and cardiovascular issues.
Dose:
70.8 mg/day of magnesium and 217.2 mg/day of potassium or control (lacidipine, a common antihypertensive medication)
Participants:
Treatment group: 35 male and female hypertensive patients with an average age of 58 years
Positive control group: 32 male and female hypertensive patients with an average age of 56 years
Control group: 147 healthy male and females with an average age of 54 years
Duration:
4 weeks
Results:
Patients taking magnesium and potassium supplements demonstrated an average significant decrease in their blood pressure levels. However, this decrease was less than what was observed in patients taking lacidipine, a common antihypertensive medication.
The researchers also observed that those taking magnesium and potassium supplements showed significantly improved flexibility of smaller arteries (arteries farther from the heart), while those taking lacidipine showed significantly improved flexibility of larger arteries (arteries closer to the heart).
Overall, magnesium and potassium mainly improved small artery flexibility, whereas lacidipine significantly improved large artery flexibility, suggesting that these two interventions may have different effects on arterial compliance based on the size of the arteries they target.
Year:
2006
Link:
Study 6
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To investigate the effects of magnesium supplementation in women with mild to moderate hypertension.
Dose:
485 mg of magnesium/day (485 mg) as magnesium aspartate-HCI or placebo
Participants:
91 women, with an average age of 57 years old
Duration:
6 months
Results:
By the end of the study, researchers observed that the group taking magnesium supplements had a significantly larger decrease in diastolic blood pressure (the pressure in your arteries when your heart is resting between beats), which was 3.4 mm Hg more compared to the placebo group. There was also a trend toward reduced systolic blood pressure (the pressure in your arteries when your heart beats).
Year:
1994
Link:
Study 7
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To investigate the effects of magnesium supplementation on blood pressure in Brazilian hypertensive patients.
Dose:
600 mg of magnesium/day (3 x 200 mg) or placebo
Participants:
15 male and female adults aged 35-65 years
Duration:
3 weeks
Results:
The study found that oral magnesium supplementation lowers blood pressure. The effect was stronger in patients with a shorter duration of high blood pressure, with around 40% reported a significant reduction of more than 10 mmHg. Magnesium supplements may be particularly effective for those with recent high blood pressure.
Year:
1995
Link:
Study 8
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To evaluate the effects of oral magnesium supplementation in individuals with metabolic syndrome and who have low levels of magnesium in the blood
Dose:
382 mg/day of elemental magnesium (30 mL of magnesium chloride
5% solution) or a placebo solution
Participants:
198 men and women with an average age of 40 years
Duration:
16 weeks
Results:
Researchers observed significant improvements in participants who received magnesium supplementation. These included a significant drop in systolic blood pressure (the top number) by 3.6 mmHg and diastolic blood pressure (the bottom number) by 5.5 mmHg compared to placebo. In addition, the researchers also observed a significant decrease in fasting blood sugar levels, significant reduction in triglyceride levels, and a significant increase in the amount of magnesium in the blood.
Year:
2017
Link:
Study 9
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To investigate the effect of magnesium treatment on blood pressure in patients with mild essential hypertension
Dose:
1-3 weeks: 15 mmol/day of magnesium or placebo
4-6 weeks: 30 mmol/day of magnesium or placebo
7-9 weeks: 40 mmol/day of magnesium or placebo
Participants:
17 mild hypertensive men and women with an average age of 50 years
Duration:
9 weeks
Results:
The study found an association between magnesium supplementation and a significant dose-dependent reduction in blood pressure in patients with mild essential hypertension. In other words, the more magnesium they took, the greater the decrease in their blood pressure. Meanwhile, the placebo group did not show any significant changes in their blood pressure.
Year:
1993
Link:
Study 10
Study type:
Pilot study
Purpose:
To evaluate the effects of magnesium in individuals with metabolic syndrome and have normal magnesium levels.
Dose:
400 mg/day of magnesium in the form of magnesium citrate or placebo
Participants:
42 men and women with metabolic syndrome and have an average age of 66 years (24 completed the study)
Duration:
12 weeks
Results:
The researchers observed a significant drop in both the top and bottom blood pressure numbers in the group taking magnesium for 12 weeks compared to their blood pressure at the beginning of the study. The top number, which shows the pressure when the heart beats, decreased from 145 to 120.8 mmHg, and the bottom number, which shows the pressure when the heart rests, went down from 85.4 to 78.5 mmHg. They also noticed a significant decrease in blood sugar levels after taking magnesium compared to the placebo group. These findings suggest that magnesium supplementation might help manage certain aspects of metabolic syndrome, especially blood pressure and blood sugar.
Year:
2021
Link:
PRODUCTS CONTAINING BLOOD PRESSURE SUPPORT
Blood Pressure Support

Blood Pressure Support & Omega 3 (Discount Price)

Blood Pressure Support - 2 Months'

Blood Pressure Support - 3 Months'

Blood Pressure Support

Blood Pressure Support With FREE Vitamin D3 & K2

Easy Sleep Scientific Studies
The effects of our Easy Sleep product have been thoroughly studied through various clinical trials and research. We've highlighted some of the most interesting scientific findings on key ingredients like lemon balm, Sensoril® ashwagandha extract, chamomile, apigenin, magnesium bisglycinate, L-theanine, and tart cherry powder.
Study 1
Study type:
Randomised, double-blind, placebo controlled trial
Purpose:
To assess the effects of lemon balm on sleep quality of postmenopausal women. Research has shown that sleep disorder is one of the most common problems in menopausal women.
Method of evaluation:
Sleep quality was assessed using a self-reported questionnaire called the Pittsburgh Sleep Quality Index, which measures seven areas: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction.
Dose:
500 mg/day lemon balm extract (2 x 250 mg capsules) or placebo
Participants:
110 postmenopausal women with an average age of 53 years
Duration:
1 month
Results:
The researchers observed a significant improvement in sleep quality in the lemon balm group compared to the control group after 1 month of intervention. These results suggest that lemon balm may be a beneficial, natural option for enhancing sleep quality over a relatively short period of time.
Year:
2020
Link:
Study 2
Study type:
Randomised, double-blind, placebo controlled trial
Purpose:
To determine the effects of lemon balm supplementation on depression, anxiety, stress, and sleep disturbances in patients with chronic stable angina, a type of chest pain that happens during physical activity or stress due to reduced blood flow to the heart.
Method of evaluation:
Sleep quality was assessed before and after the intervention using the Pittsburgh Sleep Quality Index, a self-reported questionnaire which measures seven areas: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction.
Dose:
3,000 mg/day of lemon balm (3 x 1,000 mg capsules) or placebo
Participants:
73 men and women with an average age of 58 years
Duration:
8 weeks
Results:
The researchers observed a significant decrease in the total sleep disorder score in the intervention group compared to the control group after treatment. Specifically, the intervention group demonstrated better sleep quality, longer sleep duration, and improved sleep efficiency compared to the control group.
Year:
2018
Link:
Study 3
Study type:
Randomised, double-blind, placebo controlled trial
Purpose:
To evaluate the effects of lemon balm on anxiety and sleep quality in patients undergoing coronary artery bypass surgery. Studies have shown that anxiety and sleep disorders are common following surgery, often affecting recovery and overall well-being.
Method of evaluation:
Sleep quality was assessed using a self-reported questionnaire called St. Mary’s Hospital Sleep Questionnaire, which measures the severity of sleep disorder.
Dose:
1500 mg/day lemon balm (3 x 500 mg capsules) or placebo
Participants:
80 men and women with an average age of 58 years
Duration:
7 days
Results:
The participants in the lemon balm group showed a significantly greater improvement in sleep quality and lower anxiety scores compared to the placebo group. Overall, the researchers observed that lemon balm improved sleep quality by 54% and reduced anxiety by 49% in patients after coronary artery bypass surgery.
Year:
2019
Link:
Study 1
Study type:
Randomised, placebo-controlled, crossover, and double-blind trial
Purpose:
To examine the effects of L-theanine on cognitive functions and stress-related symptoms, including sleep disorders, in healthy adults.
Method of evaluation:
Stress-related symptoms were assessed using self-reported questionnaires, including the Self-rating Depression Scale for depression, the State-Trait Anxiety Inventory for anxiety, and the Pittsburgh Sleep Quality Index for sleep quality and disturbances. The study also utilised several cognitive assessments to evaluate the effects of L-theanine on cognitive functions.
Dose:
200 mg/day of L-theanine or placebo
Participants:
30 men and women with an average age of 48 years
Duration:
4 weeks
Results:
Participants who took L-theanine demonstrated significant improvements in overall sleep quality, with less trouble falling asleep, fewer disturbances, and less need for sleep medication compared to the placebo. Additionally, cognitive skills improved, and stress-related symptoms like depression and anxiety decreased. Overall, the study suggests that L-theanine may enhance both sleep and mental performance more effectively than the placebo.
Year:
2019
Link:
Study 2
Study type:
Open, randomised, crossover intervention trial
Purpose:
To investigate the effects of known sleep-support supplements, including L-theanine, on life habits, sleep conditions, and sleep problems.
Method of evaluation:
Sleep quality was assessed using a self-reported questionnaire called the Pittsburgh Sleep Quality Index, which measures seven areas: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction.
Dose:
Treatment 1: 200 mg/day of L-theanine
Treatment 2: 111.1 mg/day of GABA
Treatment 3: 50 mg/day of Apocynum venetum leaf extract
Treatment 4: 300 mg/day of L-serine
Treatment 5: Mindfulness video
Treatment 6: Placebo
Participants:
106 male and female participants with an average age 46 years
Duration:
7 days of intervention per group
Results:
The researchers observed that L-theanine significantly improved sleep quality, especially for individuals struggling to fall asleep, stay asleep, or feel refreshed in the morning. People with good habits, such as less screen time and regular exercise, showed significantly better results. This suggests that combining physical activity and healthy lifestyle habits with L-theanine may lead to better sleep.
Year:
2023
Link:
Study 3
Study type:
Randomised, double-blind, placebo controlled, crossover trial
Purpose:
To investigate the effects of L-theanine in combination with alpha-s1-casein tryptic hydrolysate (a protein derived from cow's milk) on sleep quality in working adults with sleep disorders/insomnia.
Method of evaluation:
Sleep quality was assessed using a self-reported questionnaire called the Pittsburgh Sleep Quality Index, which measures seven areas: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction.
Dose:
50 mg/day of L-theanine with 150 mg/day of alpha-s1-casein tryptic hydrolysate (CTH) or placebo
Participants:
39 men and women with an average age of 37 years
Duration:
4 weeks
Results:
The study found a significant association between L-theanine combined with alpha-s1-casein and improved sleep quality. The participants in the L-theanine group reported falling asleep faster, sleeping longer, and having better-quality sleep, while also feeling more alert during the day. In addition, those who took the supplement slept 45 minutes longer than those who took a placebo, with the biggest improvements seen in both sleep duration and sleep quality.
Year:
2021
Link:
Study 4
Study type:
Clinical trial (uncontrolled)
Purpose:
To examine the effects of L-theanine on depressive symptoms, anxiety, sleep disturbance, and cognitive impairments in patients with major depressive disorders
Method of evaluation:
Sleep quality was assessed using a self-reported questionnaire called the Pittsburgh Sleep Quality Index, which measures seven areas: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction.
Dose:
250 mg/day L theanine tablets
Participants:
20 male and female patients with an average age of 43 years
Duration:
8 weeks
Results:
The study found that L-theanine administration is associated with a significant improvement in sleep disturbance among patients with major depressive disorder. The study also noted that some patients reported increased sleep duration and slightly increased dream activity, suggesting a positive impact on sleep patterns.
Year:
2016
Link:
Study 5
Study type:
Randomised, placebo-controlled, double-blind, crossover trial
Purpose:
To explore the sleep-improving effects of a lower dose L-theanine (100 mg/day) in middle-aged and older men.
Method of evaluation:
Sleep quality was assessed using a portable electroencephalogram (EEG) monitoring device, which measures brain activity during sleep. Participants placed sensors on their forehead and behind their ears, which tracked how long they slept, how quickly they fell asleep, time spent in deep and light sleep, and how often they woke up.
Dose:
100 mg/day of L-theanine or placebo
Participants:
24 men with an average age of 47 years
Duration:
1 week
Results:
Overall, there was no significant difference in sleep quality between the theanine and placebo groups. However, for those under 50 and participants who drank green tea less than 3-4 times a week, the researchers observed that theanine significantly improved stage 2 non-REM sleep. Stage 2 non-REM sleep helps lower heart rate, cool the body, and rest the brain, leading to more refreshing and higher-quality sleep.
Year:
2022
Link:
Study 6
Study type:
Randomised, single-blind, placebo-controlled trial
Purpose:
To evaluate the effects of a combination of magnesium, vitamins B6, B9, B12, rhodiola, and L-theanine (Mg-Teadiola) on stress and stress-related quality of life parameters, including sleep and pain perception, in chronically stressed individuals.
Method of evaluation:
Sleep quality was assessed before and after the intervention using the Pittsburgh Sleep Quality Index, a self-reported questionnaire which measures seven areas: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction.
Dose:
125 mg/d L-theanine with 150 mg/day of magnesium, 222 mg/d rhodiola, 0.7 mg vit B6, 0.1 mg of vit B9, and 1.25 mcg vit B12, or placebo
Participants:
100 chronically stressed, bu otherwise healthy men and women aged 18-65 years
Duration:
28 days
Results:
Mg-Teadiola showed potential for improving sleep-related quality of life, particularly in significantly reducing daytime dysfunction due to sleepiness in the longer term. The study also found an association between Mg-Teadiola supplementation and significant decreases in stress scores.
Year:
2022
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled clinical trial
Purpose:
To investigate the effects of ashwagandha (Sensorill®) in chronically stressed adults
Method of Evaluation:
Sleep quality and related parameters were assessed using three different measurement tools which collectively measure a comprehensive assessment of sleep quality , sleep disturbances, and overall well-being.
Dose:
125, 250, and 500 mg/d of ashwagandha (Sensorill®) capsules or placebo
Participants:
97 chronically stressed men and women with an average age of 35 years
Duration:
8 weeks
Results:
The researcher observed that participants consuming the standardised Withania somnifera extract (Sensoril) experienced positive effects on sleep quality. This improvement is linked to the extract's ability to reduce stress levels, which is known to affect sleep negatively
Year:
2024
Link:
Study 1
Study type:
Randomised controlled clinical trial
Purpose:
To investigate the effects of chamomile extract on melatonin levels in subjects suffering from insomnia and anxiety.
Dose:
15 ml/day of chamomile extract
Participants:
50 male and female participants, aged 18-60 years
Duration:
60 days
Results:
The researchers observed a significant increase in melatonin levels after treatment with chamomile extract compared to those not taking it (0.55 vs. 0.17 ng/mL). The researchers also observed significant decreases in triglyceride (a type of fat found in the blood) and cholesterol levels.
Year:
2022
Link:
Study 2
Study type:
Single-blind, randomised, controlled clinical trial
Purpose:
To evaluate the effects of chamomile extract on sleep quality among elderly people. Research has shown that insomnia tends to be more common as people age.
Method of evaluation:
Sleep quality was assessed by interviewing participants at four time points: before the treatment started, two weeks into it, right after it finished, and two weeks after it ended.
Dose:
400 mg/day of chamomile extract (2 x 200 mg capsules) or placebo (wheat flour capsules)
Participants:
60 men and women with an average age of 70 years
Duration:
28 days
Results:
At the beginning of the study, participants in both the treatment and placebo groups had poor sleep quality. However, after 28 days of treatment, those taking chamomile extract capsules reported a significant improvement in their sleep quality compared to the placebo group. Given that sleep problems are common in older adults and many sleep medications may have harmful side effects, the authors recommended chamomile extract as a safe way to help improve sleep in the elderly.
Year:
2017
Link:
Study 3
Study type:
Quasi-experimental clinical trial
Purpose:
To determine the effect of chamomile extract on sleep quality in elderly people admitted to nursing homes
Method of evaluation:
Sleep quality was assessed using a self-reported questionnaire which measures subjective sleep quality.
Dose:
400 mg/day of chamomile extract (2 x 200 mg capsules) or control (no intervention)
Participants:
77 men and women with an average age of 74 years
Duration:
4 weeks
Results:
Results of this study showed an association between 4 weeks consumption of chamomile extract and significant improvements in sleep quality.
Year:
2017
Link:
Study 4
Study type:
Single-blind, randomised, controlled clinical trial
Purpose:
To evaluate the effects of chamomile tea on sleep quality, fatigue, and depression in postpartum women
Method of evaluation:
Sleep quality, fatigue, and depression were assessed using standardised self-reported questionnaires that measured subjective sleep quality, postpartum depression, and perceived fatigue.
Dose:
2 g/day of chamomile dried flowers (in one tea bag steeped in 300 ml hot water for 10-15 minutes)
Participants:
80 postnatal women with an average age of 33 years (72 completed the study)
Duration:
2 weeks
Results:
Postnatal women who drank chamomile tea for 2 weeks saw a noticeable improvement in their sleep problems and symptoms of depression. These benefits were limited to the immediate term meaning, the effects only lasted while they were drinking the tea and did not continue once they stopped.
Year:
2015
Link:
Study 1
Study type:
Observational studies
Purpose:
To explore the association between sleep quality and specific dietary polyphenols (natural compounds found in plants that act as antioxidants) including apigenin in Italian adults.
Method of evaluation:
Sleep quality was assessed using a self-reported questionnaire called the Pittsburgh Sleep Quality Index, which measures seven areas: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction.
Participants:
1936 men and women with an average age of 50 years
Results:
The study found a significant association between apigenin and sleep quality. The researchers observed that people who consumed more apigenin were less likely to experience poor sleep. This suggests that a higher intake of apigenin may improve sleep quality. While other polyphenols were also studied, such as flavonoids and lignans, apigenin showed a more pronounced effect on sleep quality, indicating that apigenin may be more effective than its polyphenol counterparts in promoting better sleep
Year:
2020
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled, crossover clinical trial
Purpose:
To investigate the effects of tart cherry juice for the treatment of insomnia in older adults.
Method of evaluation:
Sleep quality was measured using self-reported health questionnaires and other validated questionnaires (the Insomnia Severity Index, the Epworth Sleepiness Scale, and the Pittsburgh Sleep Quality Index), which assess insomnia severity, sleepiness, and overall sleep quality.
After two weeks of drinking either cherry juice or a placebo, participants had an overnight polysomnography sleep study, which also assesses sleep quality and identifies any sleep disorders.
Dose:
480 mL/day of tart cherry juice (2 x 240 mL) or placebo
Participants:
8 men and women with an average age of 68 years
Duration:
2 weeks per treatment
Results:
The researchers observed that participants who consumed tart cherry juice experienced an increase in sleep time by 84 minutes, as measured by polysomnographic sleep study, which is a reliable method for assessing sleep quality. Sleep efficiency, or the percentage of time spent actually sleeping while in bed, also improved significantly based on the Pittsburgh Sleep Quality Index. While the study utilised other validated questionnaires to assess sleep quality, other questionnaires did not show significant differences. Overall, tart cherry juice appears to positively affect sleep quality, as evidenced by both polysomnography and the Pittsburgh Sleep Quality Index questionnaire.
Year:
2018
Link:
Study 2
Study type:
Open label clinical trial (uncontrolled)
Purpose:
To investigate the effects of a compound containing Montmorency tart cherry extract and Apocynum venetum on sleep and anxiety in adults with insomnia
Method of evaluation:
Insomnia was assessed using a self-assessed questionnaire (Insomnia Sleep Index), which measures the severity of insomnia.
Dose:
553 mg/day of tart cherry extract with 25 mg/day of Apocynum venetum
Participants:
46 men and women with an average age of 36 years
Duration:
4 weeks
Results:
The researchers observed that bedtime consumption of tart cherry extract and Apocynum venetum significantly reduced insomnia severity and anxiety scores while also improving self-reported sleep quality and daytime alertness. Furthermore, sleep quality and alertness continued to improve week by week throughout the study.
Year:
2024
Link:
Study 3
Study type:
Randomised, double-blind, placebo-controlled, crossover pilot study
Purpose:
To investigate the effects of a tart cherry juice beverage on sleep in older adults with insomnia.
Method of evaluation:
Insomnia was assessed using the Insomnia Severity Index (ISI), a validated seven-item questionnaire that measures difficulty falling asleep, staying asleep, daytime impacts, concerns about sleep, and overall satisfaction with sleep quality. In addition, participants maintained daily sleep diaries throughout the study period.
Dose:
16-ounce tart cherry juice (2 x 8 ounce) or placebo beverage
Participants:
15 men and women with insomnia, with an average age of 72 years
Duration:
8 weeks
Results:
The study found an association between a special tart cherry juice blend and significant improvements on sleep in older adults with insomnia. Specifically, the study showed a significant decrease in the time spent awake after falling asleep, and participants reported better overall sleep quality after drinking the juice compared to a placebo. Overall, the findings suggest that tart cherries may have promising benefits for improving sleep.
Year:
2010
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To investigate the effect of magnesium supplementation on insomnia in elderly.
Method of evaluation:
Insomnia was assessed using the Insomnia Severity Index (ISI), a seven-item questionnaire measuring sleep difficulties and satisfaction. Participants also kept daily sleep logs to track their sleep time.
Dose:
500 mg/d elemental magnesium (2 x 250 mg tablets) or placebo
Participants:
43 men and women with an average age of 65 years
Duration:
8 weeks
Results:
The researchers observed that participants who took magnesium supplements had significant improvements compared to the placebo group. These included significantly longer sleep time, better sleep efficiency, higher levels of serum renin and melatonin, and a significant decrease in insomnia severity, time to fall asleep, and stress levels. They also observed trends in reduced early morning awakenings and increased magnesium levels.
Year:
2012
Link:
Study 2
Study type:
Randomised, single-blind, placebo-controlled trial
Purpose:
To evaluate the effects of a combination of magnesium, vitamins B6, B9, B12, rhodiola, and L-theanine (Mg-Teadiola) on stress and stress-related quality of life parameters, including sleep and pain perception, in chronically stressed individuals.
Method of evaluation:
Sleep quality was assessed before and after the intervention using the Pittsburgh Sleep Quality Index, a self-reported questionnaire which measures seven areas: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction.
Dose:
150 mg/day of magnesium with 125 mg/d L-theanine, 222 mg/d rhodiola, 0.7 mg vit B6, 0.1 mg of vit B9, and 1.25 mcg vit B12, or placebo
Participants:
100 chronically stressed, bu otherwise healthy men and women aged 18-65 years
Duration:
28 days
Results:
Mg-Teadiola showed potential for improving sleep-related quality of life, particularly in significantly reducing daytime dysfunction due to sleepiness with longer use. The study also found an association between Mg-Teadiola supplementation and significant decreases in stress scores.
Year:
2022
Link:
Study 3
Study type:
Case histories (Case #1)
Purpose:
To determine the effects of magnesium supplementation in individuals with major depressive disorder
Dose:
300 mg/d of magnesium as glycinate and later as taurinate
Participant:
A 59-year-old male with a history of mild depression, previously managed with antidepressants, suddenly developed severe anxiety, insomnia, muscle spasms, and suicidal depression after a year of intense stress and poor dietary habits.
Results:
The subject reported that after the first night of starting magnesium, his sleep returned to near normal. Over the next four days, depression was significantly reduced for 4-6 hours after each dose, anxiety gradually faded, and headaches quickly disappeared. In the months that followed, normalcy was maintained only by frequent magnesium ingestion.
Year:
2006
Link:
PRODUCTS CONTAINING BLOOD PRESSURE SUPPORT
Easy Sleep Capsules

Tongkat Ali Scientific Studies
Numerous clinical trials have investigated the effects of tongkat ali in humans over the past 20 years. We have summarised some of the most important studies related to testosterone, sexual function, stress and energy.
Study 1
Study type:
Clinical trial (double-blind, placebo-controlled)
Dose:
600 mg/day of Tongkat Ali with 1.45% eurycomanone
Participants:
32 men aged from 20 to 30
Duration:
2 weeks
Results:
The researchers observed steroidogenic effects after Tongkat Ali supplementation. No adverse side effects were observed.
600mg/day was correlated with significant increases in testosterone (15%), free testosterone (34%) and estrogen (30%) levels. The testosterone level was raised slightly above the normal human range. The authors concluded that 600mg/day of Tongkat Ali could have a positive impact on sports performance.
Link:
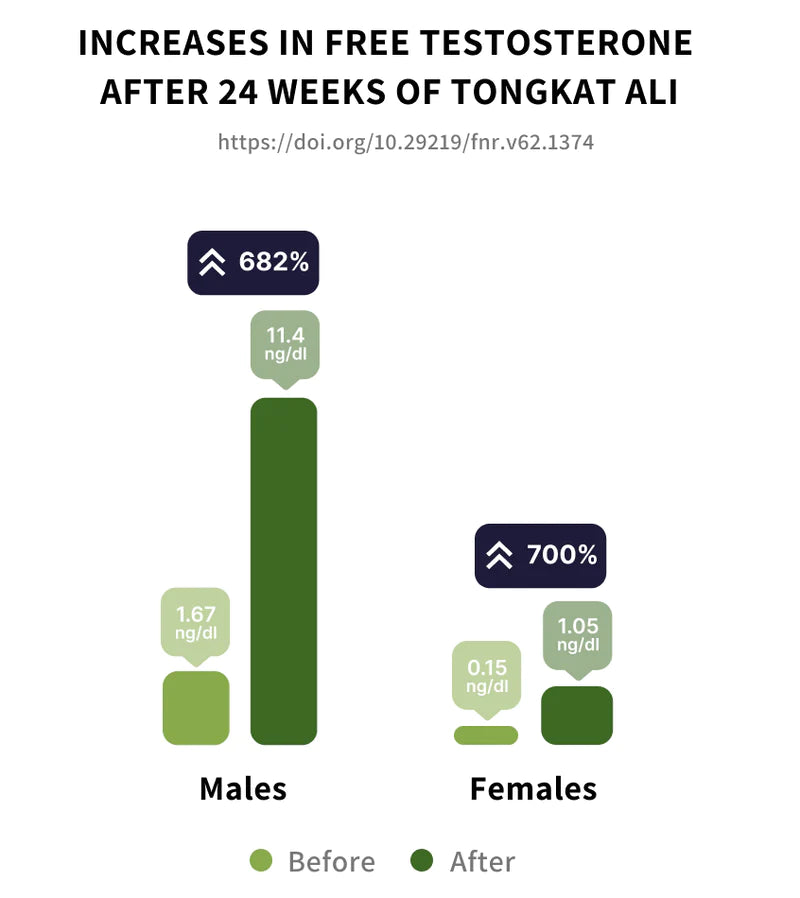
Study 2
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
50mg of Tongkat Ali per day
Additional interventions:
Multivitamin mix
Participants:
95 men and women aged from 25 to 65
Duration:
24 weeks
Results:
Tongkat Ali supplementation was associated with significant increases in free testosterone. Males experienced a 682% average increase in free testosterone. Females experienced a 700% average increase in free testosterone.
Year:
2018
Link:
Study 3
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
100mg or 200mg of Tongkat Ali per day
Participants:
106 males aged from 50 to 70 with low testosterone
Duration:
12 weeks
Results:
Tongkat ali supplementation was associated with significant increases in total testosterone. A dose of 200mg per day was associated with a 12.2% increase in total testosterone and a 16.9% increase in free testosterone.
Year:
2021
Link:
Study 4
Study type:
Randomised double-blind placebo-controlled trial
Dose:
200mg of Tongkat Ali per day
Additional interventions:
3 hour-long training sessions per week
Participants:
45 middle-aged, androgen-deficient males
Duration:
6 months (the longest trial of tongkat ali supplementation to date)
Results:
Tongkat Ali supplementation was associated with increases in total testosterone levels and improvements in erectile function.
Year:
2020
Link:
Study 5
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
200mg of Tongkat Ali per day
Participants:
26 men aged from 45 to 60 with mild erectile dysfunction
Duration:
12 weeks
Results:
Tongkat Ali supplementation was associated with a 36% increase in total testosterone. No adverse effects were observed; liver and kidney values showed no significant changes from baseline.
Link:
Study 6
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
200mg of Tongkat Ali per day
Participants:
32 men and 31 women experiencing moderate stress
Duration:
4 weeks
Results:
Tongkat Ali supplementation was associated with a 37% increase in testosterone and an improved cortisol:testosterone ratio.
Link:
STUDY 7
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
200mg, 400mg and 600mg of Tongkat Ali per day
Participants:
20 healthy males aged from 38 to 58
Duration:
2 months
Results:
Testosterone levels increased after 3 weeks of Tongkat Ali supplementation. The high dose of 600mg/d did not adversely affect liver function, renal function, blood profiles, electrolytes, cancer markers and the immune system.
Link:
Study 8
Study type:
Systematic review and meta-analysis
Studies:
9 studies in the systematic review and 5 studies in the meta-analysis
Results:
The systematic review revealed that most studies reported a significant improvement in total testosterone levels after Tongkat Ali supplementation. The meta-analysis suggested that Tongkat Ali could improve testosterone production.
Link:
Study 9
Study type:
Uncontrolled trial
Dose:
200mg of Tongkat Ali per day
Participants:
76 men with hypogonadism
Duration:
4 weeks
Results:
Tongkat Ali supplementation was associated with a 46.6% average increase in serum testosterone concentrations (from 5.66 nM to 8.3 nM). The participants also experienced improvements in erectile function and libido.
Link:
Study 10
Study type:
Uncontrolled trial
Dose:
200mg of Tongkat Ali per day
Participants:
13 physically active males and 12 physically active females aged 57 to 72
Duration:
5 weeks
Results:
Tongkat Ali was associated with significant increases in total and free testosterone concentrations and muscular force. Males experienced a 61% increase in free testosterone and a 15.1% increase in total testosterone. Females experienced a 122% increase in free testosterone and a 48.6% increase in total testosterone.
Link:
Study 11
Study type:
Animal study (male rats)
Dose:
4 fractions of Tongkat Ali at a dosage of 25 mg/kg body weight
Results:
Tongkat ali (F2) and eurycomanone significantly increased the testosterone level from the Leydig cells. The plasma luteinizing hormone and follicle stimulating hormone were also higher than the control group.
Link:
Study 12
Study type:
Cellular study (testicular interstitial cells)
Dose:
100μL solution of Eurycomanone at 0.1, 1.0 and 10μM
Results:
Eurycomanone significantly increased testosterone production dose-dependently at 0.1, 1.0 and 10.0 μM.
Link:
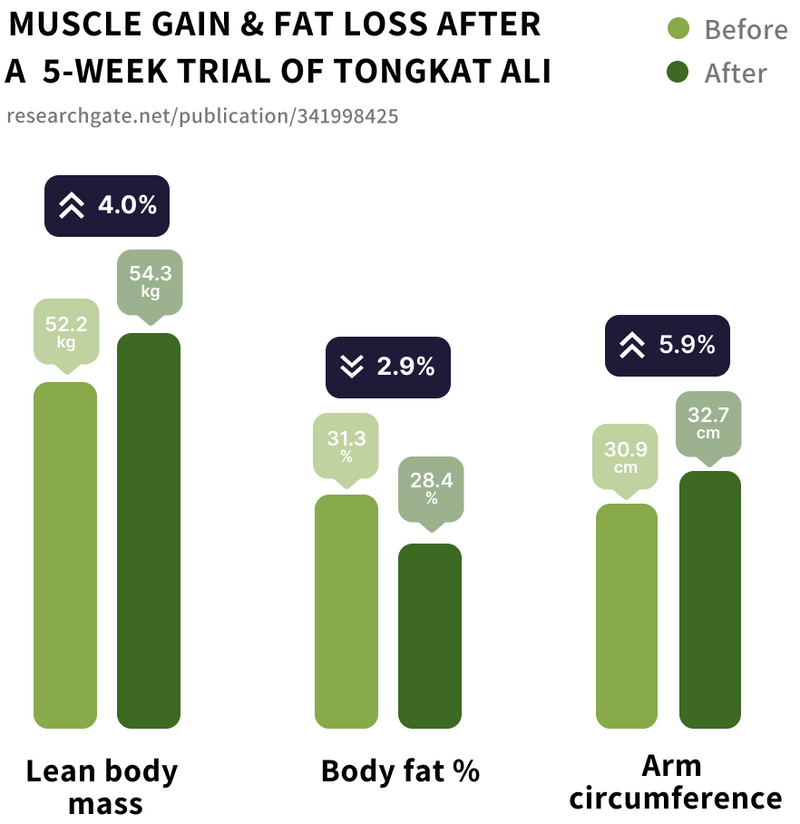
Study 1
Study type:
Placebo-controlled clinical trial
Dose:
100mg per day
Additional interventions:
Intense strength training on alternate days
Participants:
14 healthy men
Duration:
5 weeks
Results:
Tongkat Ali supplementation was associated with:
• 4.1% Increase in lean body mass (from 52.26kg to 54.39kg)
• 2.86% reduction in body fat (from 31.3% to 28.44%)
• 6.8% increase in strength
• 1.8cm increase in mean arm circumference (from 30.87cm to 32.67cm)
Link:
Study 2
Study type:
Randomised placebo-controlled trial
Dose:
250mg of Tongkat Ali per day
Additional interventions:
High-intensity resistance training; 2500mg of branched-chain amino acids; 1020mg of beta-alanine.
Participants:
32 young males with at least 1 year of experience in squatting, bench pressing and deadlifting.
Duration:
4 weeks
Results:
Tongkat Ali supplementation was associated with large strength increases in bench pressing (102kg to 108kg), squatting (120kg to 133kg) and deadlifting (158kg to 172kg), corresponding to an overall strength increase of 33kg. The placebo group experienced a much lower overall strength increase (24kg).
Year:
2015
Link:
Study 3
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
300mg
Participants:
40 men aged 30 to 35
Duration:
12 weeks
Results:
Tongkat Ali supplementation was associated with a strength increase of around 14kg in the back and leg muscles compared to the placebo group.
Year:
2013
Link:
Study 4
Study type:
Clinical trial (uncontrolled)
Dose:
200mg per day
Participants:
13 physically active males and 12 physically active females aged 57 to 72
Duration:
5 weeks
Results:
Tongkat Ali supplementation was associated with a strength increase of 16.6% in men and 13.7% in women after 5 weeks. These increases in muscle strength indirectly reflected an increase in muscle mass.
* Strength was measured with a hand dynamometer.
Year:
2013
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
300mg of Tongkat Ali per day
Participants:
109 healthy men aged from 30 to 55
Duration:
12 weeks
Results:
Tongkat Ali was associated with significant improvements in erectile function, sexual libido and overall sexual satisfaction scores. Tongkat Ali was also associated with higher sperm motility and semen volume. The study also indicated that healthy, younger males may require doses higher than 300mg per day to increase testosterone levels.
Year:
2012
Link:
Study 2
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
200mg per day
Participants:
26 men aged from 45 to 60 with mild erectile dysfunction
Duration:
12 weeks
Results:
Tongkat Ali supplementation was associated with significant improvements in the frequency of sexual performance, sexual satisfaction, penile erection and hardness, and overall sexual wellbeing. Participants also experienced an average increase in total testosterone by 36%. No adverse effects were observed; liver and kidney values showed no significant changes from baseline.
Year:
2014
Link:
Study 3
Study type:
Clinical trial (uncontrolled)
Dose:
200mg per day
Participants:
75 males (aged 33 on average) with idiopathic infertility
Duration:
3 months
Results:
Tongkat Ali supplementation was associated with significantly improved sperm quality (higher semen concentration, sperm motility and sperm concentration) and 11 spontaneous pregnancies.
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
50mg of Tongkat Ali per day
Additional interventions:
Multivitamin mix
Participants:
95 men and women aged from 25 to 65
Duration:
24 weeks
Results:
Tongkat Ali supplementation was associated with significant improvements in reported stress levels, quality-of-life, mood, vigour, emotional well-being and cognition.
Year:
2018
Link:
Study 2
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
200mg per day
Participants:
32 men and 31 women experiencing moderate stress
Duration:
4 weeks
Results:
Tongkat Ali supplementation was associated with significant improvements in tension (-11%), confusion (-15%) and anger (-21%). Tongkat Ali was also associated with a reduction in cortisol (−16%) and increased testosterone (+37%). Note that cortisol levels generally increase in response to stress.
Year:
2022
Link:
TURKESTERONE SCIENTIFIC STUDIES
Several studies have investigated the effects of turkesterone. We have summarised the most interesting results.
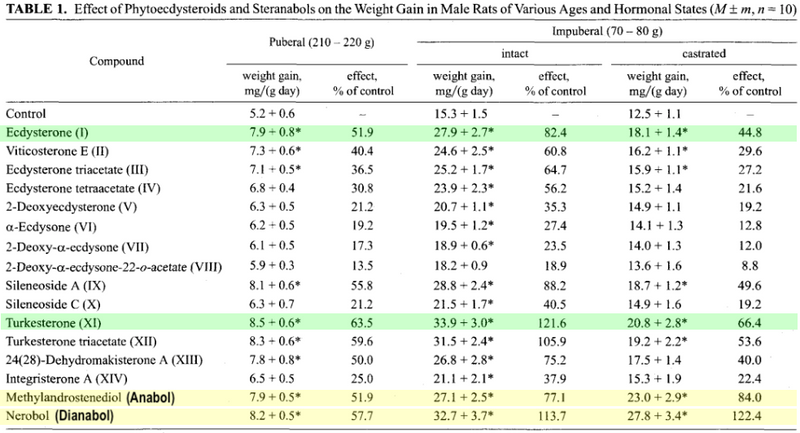
Study 1: Turkesterone outperforms anabolic steroids
Study type:
Rodent study (male rats)
Purpose
To compare the anabolic activity of phytoecdysteroids and steranabols (anabolic steroids). Specifically, turkesterone and ecdysterone were compared with methyandrostenediol (Anabol) and nerobol (Dianabol). The effect of phytoecdysteroids upon protein-anabolic processes were judged by changes in body weight and the weight of internal organs and skeletal muscles.
Dose:
5 mg/kg/day of phytoecdysteroids for 10 days or 10 mg/kg/day of Dianabol/Anabol for 10 days.
Results:
The results showed that the anabolic activity of turkesterone outperformed anabolic steroids.
As visible in the table pictured, puberal and intact impuberal rodents experienced greater weight gain from turkesterone than from nerabol (Dianabol) and methylandrostenediol (Anabol).
These results are remarkable for several reasons:
1. Dianabol and Anabol are potent anabolic steroids that bodybuilders have used for decades.
2. Anabol and Dianabol generally lead to artificial weight gain due to water retention, unlike turkesterone and ecdysterone.
3. Turkesterone and ecdysterone stimulated protein synthesis without adverse effects on the endocrine system.
Link:
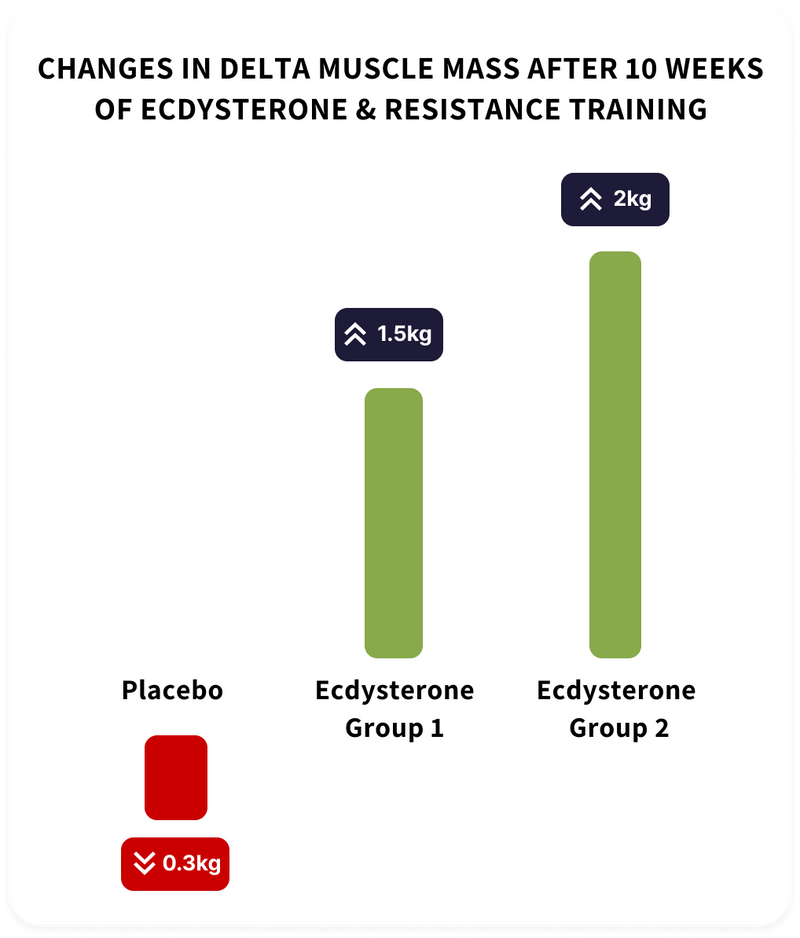
Study 2
Study type:
Non-randomised placebo-controlled clinical trial
Purpose:
To measure the effects of ecdysteroids (ecdysterone) on sports performance and body composition.
Interventions:
Ecdysterone supplement and 3 sessions of resistance training per week
Participants:
46 young men with at least 1 year of experience in weight-lifting (bench press, deadlift and squat)
Duration:
10 weeks
Results:
Compared to the placebo group, ecdysterone was correlated with significantly larger increases in muscle mass. The placebo group actually lost muscle mass.
Ecdysterone supplementation was also correlated with a significant increase in bench press and squat performance compared to the placebo group.
Importantly, biomarkers for liver or kidney toxicity did not increase after ecdysterone supplementation.
Year:
2019
Study sponsor:
WADA
Link:
Study 3
Study type:
Animal & cellular study
Purpose
To elucidate the anabolic potency of ecdysterone (an ecdysteroid similar to turkesterone) in comparison to other known anabolic agents and to support the hypothesis of ERβ mediated action by in-silico modelling.
Dose:
5 mg/kg/day of phytoecdysteroids for 10 days or 10 mg/kg/day of methylandrostenediol (Anabol) or nerobol (Dianabol) for 10 days.
Results:
In the rodent study, ecdysterone exhibited a strong hypertrophic effect on the fibre size of the soleus muscle that was found to be even stronger than dianabol (metandienone), trenbolox (estradienedione), and SARMS1, all administered with the same dose (5 mg/kg for 21 days).
In the cellular study, ecdysterone (1 µM) induced a significant increase of the diameter of myotubes (a cell found in muscle fibres) comparable to dihydrotestosterone (1 µM) and IGF1 (Insulin-Like Growth Factor, 1.3 nM).
Conclusion:
The anabolic potency of ecdysterone was comparable or even higher than the anabolic androgenic steroids, SARMs or IGF-1.
Year
2015
Link:
Study 4
Study type:
Cellular study
Purpose
To study the mechanism of action of phytoecdysteroids in mammalian tissue.
Results:
In human primary myotubes (cells found in muscle fibres), phytoecdysteroids increased protein synthesis by up to 20%.
Year
2008
Link:
Study 5
Study type:
Cellular study
Purpose
To evaluate whether phytoecdysteroids increase protein synthesis.
Results:
20-hydroxyecdysone (ecdysterone), a common phytoecdysteroid in both insects and plants, increased protein synthesis in myotubes (cells found in muscle fibres) by up to 16%.
Year
2010
Link:
Products Containing Turkesterone
Turkesterone 10% Standardisation
Just £1 per day

-
 Muscle GrowthMuscle Growth Rating 10/10.010/10
Muscle GrowthMuscle Growth Rating 10/10.010/10 -
 Strength GainsStrength Gains Rating 10/10.010/10
Strength GainsStrength Gains Rating 10/10.010/10 -
 Erection ImprovementErection Improvement Rating 3/10.03/10
Erection ImprovementErection Improvement Rating 3/10.03/10 -
 Libido BoostLibido Boost Rating 3/10.03/10
Libido BoostLibido Boost Rating 3/10.03/10 -
 Stress ReductionStress Reduction Rating 2/10.02/10
Stress ReductionStress Reduction Rating 2/10.02/10
CISTANCHE SCIENTIFIC STUDIES
We have summarised the most interesting studies of Cistanche related to depression, cognitive health, testosterone levels, sperm quality, erectile function and hair loss.
Study 1
Study type:
Animal study (rats)
Purpose:
To determine the effects of Cistanche tubulosa extract on hormone levels and testicular steroidogenic enzymes in rats.
Results:
Cistanche tubulosa increased blood levels of testosterone and progesterone. It also increased sperm count 2.3 to 2.7-fold, increased sperm motility 1.3 to 1.4-fold, decreased abnormal sperm 0.76 to 0.6-fold, and increased the expression (activation) of the steroidogenic enzymes CYP11A1, CYP17A1, and CYP3A4. Steroidogenic enzymes are enzymes that are involved in steroid biosynthesis.
Conclusion:
Cistanche tubulosa extract decreased abnormal sperm and increased testosterone, progesterone, sperm count, sperm motility and the activation of enyzmes that are involved in the synthesis of steroid hormones.
Year
2016
Link:
Study 2
Study type:
Cellular and animal study (diabetic rats)
Purpose:
To investigate the anti-inflammatory and protective effects of echinacoside in Cistanche tubulosa extracts on the reproductive system of male diabetic rats (echinacoside is an active component of Cistanche tubulosa).
Results:
Rats treated with various concentrations of echinacoside in Cistanche tubulosa extracts exhibited higher levels of testosterone than the non-treated diabetic rats. Similarly, the results of the cellular study indicated that eichinacoside restored the testosterone synthesis pathway.
Echinacoside also improved blood sugar levels, insulin resistance (the responsiveness of cells to insulin), leptin resistance (the body’s responsiveness to leptin, a hormone that signals the brain to stop eating when enough body fat has been stored), and lipid peroxidation (a process that causes oxidative deterioration of lipids, the fundamental building block of cells).
Summary:
Cistanche tubulosa and echinacosides increased testosterone and displayed anti-inflammatory and antioxidant benefits.
Year
2018
Link:
Study 3
Study type:
Animal study (rats)
Purpose:
To investigate the proactive effects of Cistanche tubulosa and echinacoside (an active ingredient of Cistanche) against testicular and sperm toxicity.
Results:
Cistanche tubulosa and echinacoside normalised blood testosterone and reversed abnormalities in sperm characteristics and testicular structure. Cistanche and echinacoside also increased steroidogenic enzymes (enzymes involved in the production of steroid hormones), indicating that testosterone production was improved.
The results are especially interesting as the testicular toxicity was induced in the rats via a toxic chemical called bisphenol A (BPA) which has been used to make many plastics such as food containers since the 1950s. BPA is known to be an endocrine-disruptor, meaning that it interferes with the body’s hormones. Studies have indicated that BPA has estrogen-like activity and exhibits inhibitory effects on testosterone synthesis and developmental toxicity in the reproductive organs. Daily BPA exposure is unavoidable for most people.
This study indicated that Cistanche may help to protect against the potential effects of daily BPA exposure.
Conclusion:
Cistanche tubulosa and echinacoside improved testicular and sperm damage in rats.
Year
2016
Link:
Study 4
Study type:
Animal study (rats)
Purpose:
To investigate the protective effects of echinacoside (echinacoside is an active component of Cistanche tubulosa) against infertility in rats.
Results:
Echinacoside increased sperm quantity and protected against oligoasthenospermia (low sperm count and sperm motility, a primary cause of male infertility). Echinacoside blocked androgen-receptor activity (the binding sites of testosterone) in the hypothalamus, thereby increasing testosterone.
Conclusion:
Echinacoside increased sperm quantity and protected against low sperm count and low sperm motility. It also increased levels of testosterone and luteinizing hormone.
Year
2018
Link:
Study 5 (Growth hormone)
Study type:
Animal study (rats)
Results:
Eichanoside from Cistanche tubulosa stimulated growth hormone secretion via activation of the ghrelin receptor. Note that the ghrelin receptor is the target of performance enhancing drugs and SARMS (drugs similar to anabolic steroids).
Conclusion:
Eichanoside from Cistanche tubulosa stimulated the secretion of growth hormone.
Year
2016
Link:
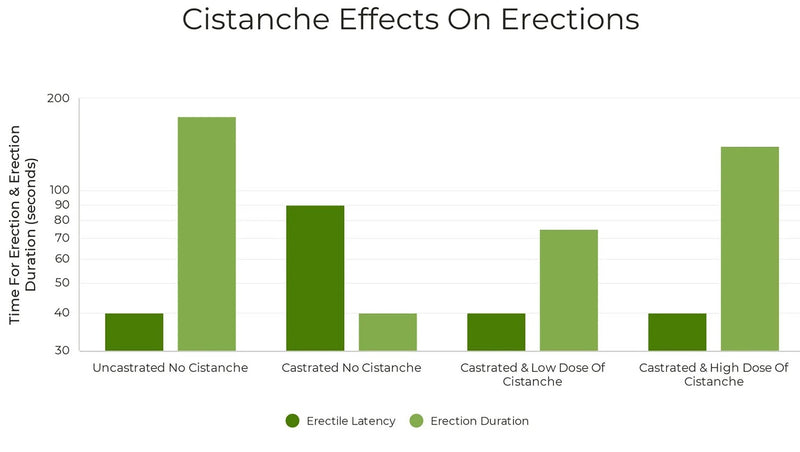
Study 1
Study type:
Animal study (rats)
Purpose:
To examine the effects of Cistanche deserticola extract on penis erectile response.
Results:
The findings indicated that Cistanche deserticola shortened erectile latency and prolonged erectile duration to minimise the negative effects of castration.
The results also indicate that Cistanche deserticola regulated the blood concentration of luteinizing hormone to normal levels. In men, luteinizing hormone stimulates Leydig cells in the testes to produce testosterone. This acts to support sperm production.
Conclusion:
In castrated rats, the results indicated that Cistanche deserticola decreased the time taken to get an erection and increased the duration of erections.
Year
2016
Link:
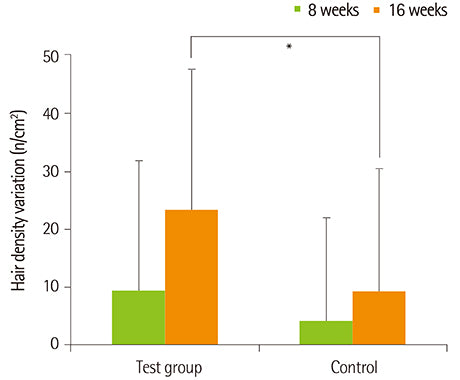
Study 1
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To investigate the efficacy of Cistanche tubulosa and Laminaria japonica in preventing hair loss and promoting scalp health.
Number of participants:
94
Duration:
16 weeks
Results:
Researchers observed a statistically significant increase in the hair density of the test group after 16 weeks of Cistanche tubulosa and Laminaria japonica. Researchers also observed a statistically significant increase in hair diameter in the test group compared to control group at week 16. The test group also experienced greater improvements in dandruff compared to the control.
Conclusion:
Cistanche tubulosa extract was associated with improvements in patterned hair loss, dandruff and inflammation of the scalp.
Year
2015
Link:
Study 1 (Depression)
Study type:
Animal study (mice)
Purpose:
To examine the antidepressant effects of Cistanche tubulosa.
Results:
The results showed promising effects as a potential therapy for depression.
The mice treated with Cistanche displayed:
1. An improved ability of spatial learning and memory.
2. Downregulated monoamine oxidase activity. It is important to note that monoamine oxidase inhibitors are used as antidepressant drugs. Monoamine oxidase is an enzyme that serves to inactivate monoamine neurotransmitters such as dopamine and serotonin.
3. Upregulated dopamine concentration in the brain (dopamine is a chemical involved in feelings of pleasure and motivation).
4. Downregulated blood concentration of corticosterone, indicating that the mice had reduced stress levels (corticosterone is a biomarker of stress levels).
Conclusion:
Cistanche improved markers of depression.
Year
2017
Link:
Study 2 (Depression)
Study type:
Animal study (rats)
Purpose:
To examine the impact of Cistanche tubulosa on markers of depression.
Results:
Cistanche tubulosa extract significantly improved depression-like behaviours in rats under chronic unpredictable stress. Cistanche restored levels of serotonin (serotonin plays a key role in mood stabilisation and sleep). Cistanche also restored BDNF protein expression (loss of BDNF is thought to be involved in depression).
Year
2018
Link:
Study 3 (Depression)
Study type:
Animal study (rats)
Purpose:
To explore the active components of Cistanche tubulosa against depression
Results:
Molecules called glycosides from the stems of Cistanche tubulosa improved depression-like behaviours in rats.
Year
2021
Link:
Study 4 (Alzheimer's disease)
Study type:
Animal study (Alzheimer’s disease-like rat model)
Propose:
To evaluate the effect of Cistanche in rats with Alzheimer’s disease-like pathology
Results:
More specifically, Cistanche reversed cholinergic and dopaminergic dysfunction in rats. The results are promising as Alzheimer’s disease is associated with cholinergic and dopaminergic dysfunction.
Conclusion:
Cistanche reversed dysfunction associated with Alzheimer’s disease.
Year
2014
Link:
Study 5 (Parkinson's disease)
Study type:
Animal study (rodent model of Parkinson’s disease)
Purpose:
To identify potential therapeutic effects of Cistanche on Parkinson’s disease.
Results:
Cistanche had protective effects on dopaminergic neurons in a rodent model of Parkinson’s disease. This is promising as Parkinson’s disease mainly affects the dopamine-producing (“dopaminergic”) neurons.
Conclusion:
Cistanche treatment resulted in protective effects against Parkinson’s disease.
Year
2016
Link:
Products Containing Cistanche
Cistanche Capsules
Just £1 per day

Fadogia Agrestis Scientific Studies
We have summarised the most interesting studies on Fadogia agrestis.
Study 1 (Testosterone & Libido)
Study type:
Animal study (rats)
Study length:
5 days
Results:
The various doses of Fadogia agrestis (18 mg/kg, 50 mg/kg and 100 mg/kg) produced two-, three- and six-fold increases in blood testosterone compared with the control.
All doses of Fadogia agrestis significantly prolonged the ejaculatory latency and improved markers of libido. It increased mount and intromission frequency (higher intercourse frequency) and reduced mount and intromission latency (reduced time before intercourse).
Conclusion:
All doses of Fadogia agrestis increased testosterone levels, delayed ejaculation, increased intercourse frequency and improved markers of libido. 100 mg/kg of Fadogia agrestis increased blood testosterone levels by 600%.
Year
2005
Link:
Study 2 (Erectile Dysfunction)
Study type:
Animal study (rats)
Results:
Fadogia agrestis restored the NO/cGMP pathway (a mediator of erections) and key enzymes in the penile and testicular tissues of male rats.
Conclusion:
Fadogia agrestis may help improve erectile dysfunction, although clinical trials are needed.
Year
2023
Link:
Products Containing Fadogia
Fadogia Agrestis Capsules
Just £1 Per Day

-
 Testosterone BoostTestosterone Boost Rating 8/10.08/10
Testosterone BoostTestosterone Boost Rating 8/10.08/10 -
 Muscle GrowthMuscle Growth Rating 7/10.07/10
Muscle GrowthMuscle Growth Rating 7/10.07/10 -
 Strength GainsStrength Gains Rating 7/10.07/10
Strength GainsStrength Gains Rating 7/10.07/10 -
 Erection ImprovementErection Improvement Rating 7/10.07/10
Erection ImprovementErection Improvement Rating 7/10.07/10 -
 Libido BoostLibido Boost Rating 7/10.07/10
Libido BoostLibido Boost Rating 7/10.07/10 -
 Stress ReductionStress Reduction Rating 8/10.08/10
Stress ReductionStress Reduction Rating 8/10.08/10
ECDYSTERONE SCIENTIFIC STUDIES
Several studies have investigated the effects of ecdysterone. We have summarised the most interesting results.
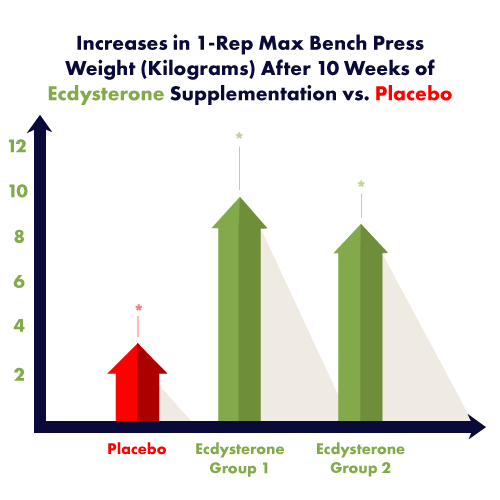
Study 1
Study type:
Non-randomised placebo-controlled clinical trial
Purpose:
To measure the effects of ecdysteroids (ecdysterone) on sports performance and body composition.
Interventions:
Ecdysterone supplement and 3 sessions of resistance training per week
Participants:
46 young men with at least 1 year of experience in weight-lifting (bench press, deadlift and squat)
Duration:
10 weeks
Results:
Compared to the placebo group, ecdysterone was correlated with significantly larger increases in muscle mass. The placebo group actually lost muscle mass.
Ecdysterone supplementation was also correlated with a significant increase in bench press and squat performance compared to the placebo group.
Importantly, biomarkers for liver or kidney toxicity did not increase after ecdysterone supplementation.
Year:
2019
Study sponsor:
WADA
Link:

Study 2
Study type:
Animal & cellular study
Purpose
To elucidate the anabolic potency of ecdysterone in comparison to other known anabolic agents and to support the hypothesis of ERβ mediated action by in-silico modelling.
Dose:
5 mg/kg/day of phytoecdysteroids for 10 days or 10 mg/kg/day of methylandrostenediol (Anabol) or nerobol (Dianabol) for 10 days.
Results:
In the rodent study, ecdysterone exhibited a strong hypertrophic effect on the fibre size of the soleus muscle that was found even stronger compared to dianabol (metandienone), trenbolox (estradienedione), and SARMS1, all administered in the same dose (5 mg/kg body weight, for 21 days).
In the cellular study, ecdysterone (1 µM) induced a significant increase of the diameter of myotubes (a cell found in muscle fibres) comparable to dihydrotestosterone (1 µM) and IGF1 (Insulin-Like Growth Factor, 1.3 nM).
Conclusion:
The anabolic potency of the ecdysterone was comparable or even higher than the anabolic androgenic steroids, SARMs or IGF-1.
Year
2015
Link:

Study 3
Study type:
Animal study (male rats)
Purpose
To compare the anabolic activity of phytoecdysteroids and steranabols (anabolic steroids). Specifically, ecdysterone and turkesterone were compared with methyandrostenediol (Anabol) and nerobol (Dianabol). The effect of phytoecdysteroids upon protein-anabolic processes were judged by changes in body weight and the weight of internal organs and skeletal muscles.
Dose:
5 mg/kg/day of phytoecdysteroids for 10 days or 10 mg/kg/day of methylandrostenediol (Anabol) or nerobol (Dianabol) for 10 days.
Results:
The results showed that the anabolic activity of phytoecdysteroids outperformed anabolic steroids.
As visible in the table below, puberal rodents experienced similar weight gain from ecdysterone similar to the anabolic steroid Anabol (methylandrostenediol). Intact impuberal rodents experienced greater weight gain from ecdysterone than from Anabol.
These results are significant for several reasons:
- Anabol leads to artificial weight gain due to water retention, unlike ecdysterone.
- The phytoecdysteroids (ecdysterone and turkesterone) stimulated protein synthesis without adverse effects on the endocrine system.
Year
2000
Link:
Study 4
Study type:
Cellular study
Purpose
To study the mechanism of action of phytoecdysteroids in mammalian tissue.
Results:
In human primary myotubes (cells found in muscle fibres), phytoecdysteroids increased protein synthesis by up to 20%.
Year
2008
Link:
Study 5
Study type:
Cellular study
Purpose
To evaluate whether phytoecdysteroids increase protein synthesis.
Results:
20-hydroxyecdysone (ecdysterone), a common phytoecdysteroid in both insects and plants, increased protein synthesis in myotubes (cells found in muscle fibres) by up to 16%.
Year
2010
Link:
Products Containing Ecdysterone
Ecdysterone 90% Tablets

-
 Muscle GrowthMuscle Growth Rating 10/10.010/10
Muscle GrowthMuscle Growth Rating 10/10.010/10 -
 Strength GainsStrength Gains Rating 10/10.010/10
Strength GainsStrength Gains Rating 10/10.010/10 -
 Erection ImprovementErection Improvement Rating 3/10.03/10
Erection ImprovementErection Improvement Rating 3/10.03/10 -
 Libido BoostLibido Boost Rating 3/10.03/10
Libido BoostLibido Boost Rating 3/10.03/10 -
 Stress ReductionStress Reduction Rating 2/10.02/10
Stress ReductionStress Reduction Rating 2/10.02/10
Horny Goat Weed Scientific Studies
We have summarised some of the most interesting scientific studies on horny goat weed and icariin. Icariin is the main active component of horny goat weed.
Study 1
Study type:
Rats with erectile dysfunction due to nerve injury
Purpose
To evaluate the effects of icariin (icariin is believed to be the main active component of horny goat weed) on penile blood flow and tissues in cavernous nerve-injured rats (the cavernous nerve is the main autonomic nerve regulating penile erection).
Dose:
1, 5, and 10 mg/kg of icariin
Duration:
4 weeks
Results:
Daily treatment with low-dose icariin for 4 weeks improved penile blood flow dynamics in rats with a cavernous nerve injury (the nerve that regulates erections).
Compared to the control, the researchers detected significantly higher nNOS in the penile tissue. Note that nNOS, or neuronal NO synthase, generates nitric oxide which initiates penile erections.
Conclusion:
This study indicates that icariin (found in horny goat weed) may improve penile blood flow. Note that penile blood flow is essential for erections. Clinical studies are needed to investigate its efficacy in humans.
Year
2010
Link:
Study 2
Study type:
Rats with erectile dysfunction due to nerve injury
Purpose
To investigate the effect of icariin on erectile function and the expression of nitric oxide synthase* in castrated rats.
*Nitric oxide synthase generates nitric oxide that initiates penile erections.
Dose:
1 mg/kg/day and 5 mg/kg/day
Duration:
4 weeks
Results:
Several markers of erectile function improved in most treatment groups:
1. In 2 out of 3 treatment groups, the expression (activation) of two forms of nitric oxide synthase significantly improved in the erectile tissue of rats. Note that nitric oxide synthase generates nitric oxide which initiates penile erections.
2. In 1 of the treatment groups, phosphodiasterase-5 was significantly decreased. Note that phosphodiesterase-5 interferes with blood flow to the penis that is needed for an erection.
Conclusion:
Oral treatment with icariin for 4 weeks potentially improves erectile function in rodents.
Year
2005
Link:
Study 3
Study type:
Rats with erectile dysfunction
Purpose
To investigate the effects of orally administered icariin on the expression (levels) of nitrogen oxide synthase* in the erectile tissue of rats with erectile dysfunction.
Dose:
5 mg/kg/day or 10 mg/kg/day
Duration:
30 days
Results:
Chronic oral treatment with icariin increases erectile function and restores nitric oxide synthase* in the erectile tissue of rats.
Year
2004
Link:
https://pubmed.ncbi.nlm.nih.gov/15329286/
*(note that nitric oxide synthase generates nitric oxide which initiates penile erections)
Study 4
Study type:
Laboratory study
Purpose
To investigate the effect of icariin on phosphodiesterase-5
Results:
Icariin exhibited inhibitory effects on all forms of phosphodiesterase-5.
Year
2006
Link:
Study 1 (estrogen, cholesterol and triglyceride levels in postmenopausal women)
Study type:
Randomised, double-blind, placebo-controlled clinical trial
Purpose:
To evaluate the effects of dried Epimedium extract on blood lipid levels and sex hormone levels (estradiol, progesterone and testosterone).
Background information:
Horny goat weed contains phytoestrogens, compounds that act like the hormone estrogen.
Dose:
300mL of hot water Epimedium extract (with 25% icariin)
Participants:
90 postmenopausal women
Duration:
6 months
Results:
Epimedium water extract was associated with a significant increase in blood levels of estradiol (an estrogen hormone) compared with the pre-treatment level. It was also associated with a significant decrease in total cholesterol and total triglyceride levels.
Year
2008
Link:
Study 2 (Bone Health)
Study type:
Randomised, double-blind, placebo-controlled clinical trial
Purpose:
To investigate the effect of the phytoestrogen flavonoids derived from Epimedium brevicornum maxim (horny goat weed) on bone loss in postmenopausal women.
Dose:
60mg icariin (with 15mg Daidzein and 3mg Genistein) paired with 300mg elemental calcium daily
Participants:
85 healthy late postmenopausal women
Duration:
24 months
Results:
Phytoestrogen flavonoids from horny goat weed were associated with preventative effects on bone loss in late postmenopausal women.
Year
2007
Link:
BEETROOT SCIENTIFIC STUDIES
Dozens of clinical trials have investigated the effects of beetroot supplementation. We have summarised some the most interesting scientific studies.
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
250 mL/day of beetroot juice (containing ~6.4mmol of nitrate) or a placebo (250 mL of nitrate-depleted beetroot juice)
Participants:
64 hypertensive men and women aged 18 to 85
Duration:
4 weeks
Results:
Daily supplementation with beetroot juice (dietary nitrate) was associated with a reduction in blood pressure. Specifically, the mean 24h ambulatory blood pressure decreased by 7.7/5.2 mmHg and the mean clinical blood pressure decreased by 7.7/2.4 mmHg. These reductions are clinically significant as they resemble the average reduction in blood pressure after a single anti-hypertensive drug at standard dose (9.1/5.5 mmHg). The authors of the study suggested that dietary nitrate could be used as an adjunctive therapy for managing hypertension.
Year:
2014
Link:
Study 2
Study type:
Randomised, double-blind, placebo-controlled feasibility trial
Dose
Participants were randomly assigned to consume either:
1. High-nitrate beetroot juice (∼400 mg nitrate) and folic acid (∼5 mg folic acid) (N+F)
2. High-nitrate beetroot juice and placebo (N+P)
3. Nitrate-depleted beetroot juice and placebo (P+P)
Participants:
47 men and women aged 50 to 70 with BMIs between 26 and 29
Duration:
60 days
Results:
Supplementation with beetroot juice (dietary inorganic nitrate) was associated with lower systolic and diastolic blood pressure. At the end of the study, 24h systolic blood pressure dropped by −10.8, −6.1 and −0.3 mmHg in the N+P, N+F, and P+P groups, respectively. There was a significant decrease in 24h diastolic blood pressure in the N+P group (−5.4 mmHg), whereas changes were not significant in the N+F (−1.8 mmHg) and P+P (1.6 mmHg) groups.
Year:
2020
Link:
Study 3
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
314 mM of nitrate tablets or a placebo (nitrate-free tablets). The nitrate tablets consisted of nitrate-rich beetroot extract 20mg, thiamine mononitrate 90mg, potassium nitrate 480mg, ascorbic acid 150mg, folic acid 200mcg, methylcobalamin 200mcg, calcium 115mg, pomegranate fruit extract 5mg and green coffee bean extract 115mg. Ascorbic acid was added to facilitate NO bioavailability.
Participants:
67 hypertensive men and women with a mean age of 59
Duration:
12 weeks
Results:
Nitrate tablets were associated with a larger average reduction in systolic blood pressure than the placebo (-12.5 vs -6.19 mmHg at the end of the study). Nitrate tablets were associated with a reduction in diastolic blood pressure (-4.7 mmHg on average), whilst no significant reduction was observed in the placebo group. Endothelial function also improved by 0.8 after 12 weeks (compared to a 0.1 improvement in the placebo group). The authors concluded that endothelial function improved robustly, reducing both systolic and diastolic blood pressure in hypertensive individuals.
Year:
2020
Link:
Study 4
Study type:
Randomised, single-blind, cross-over postprandial trial (pilot study)
Dose:
In study 1, participants were randomly assigned to consume either 0, 100, 250 or 500mL of beetroot juice (each having a nitrate concentration of <0.5, 2.3, 5.7 and 11.4 mmol respectively). In study 2, participants were randomly assigned to consume either 200g of unfortified bread (<0.5 mmol of nitrate) or 200g of bread fortified with white or red beetroot (1.6 and 1.8 mmol of nitrate respectively). All participants consumed a low-nitrate/nitrite diet for 1 day before each study.
Participants:
18 healthy normotensive males in study 1 and 14 healthy normotensive males in study 2.
Duration:
Acute: blood pressure was measured over a 24h period following the consumption of beetroot juice, beetroot-fortified bread and the controls.
Results:
Supplementation with beetroot juice or beetroot-fortified bread was associated with larger reductions in blood pressure than the control. The peak reduction in blood pressure occurred after 2-3 hours:
- 100mL of beetroot juice was associated with a 13.1/16.6 mmHg reduction in blood pressure.
- 250mL of beetroot juice was associated with a 20.5/14.6 mmHg reduction in blood pressure.
- 500mL of beetroot juice was associated with a 22.2/18.3 mmHg reduction in blood pressure.
- Bread fortified with white beetroot was associated with a 19.3/16.5 mmHg reduction in blood pressure.
- Bread fortified with red beetroot was associated with a 23.6/23.2 mmHg reduction in blood pressure.
These results suggest that nitrate in beetroot may significantly help to reduce blood pressure. The reductions in blood pressure after the consumption of fortified bread suggest that processed beetroot may lower blood pressure to a similar degree as unprocessed beetroot. Thus, beetroot supplements may help to reduce overall blood pressure.
Year:
2012
Link:
Study 5
Study type:
Randomised non-blinded postprandial trial
Dose:
The experimental group consumed 500mL of beetroot juice with a mean nitrate concentration of 45.0±2.6 mMol/L (2.79g/L). The control group consumed water.
Participants:
14 healthy subjects
Duration:
Acute: blood pressure was measured over a 24h period following the consumption of beetroot juice.
Results:
Beetroot juice supplementation (dietary nitrate) was associated with larger reductions in blood pressure than the control. Specifically, blood pressure dropped by 10.4/8 mmHg after 2.5 hours compared to the control. The drop in blood pressure was correlated with increases in plasma nitrite concentration. This suggests that dietary nitrate may underlie the beneficial effects of beetroot. After 24 hours, systolic blood pressure was 4.4 mmHg lower with beetroot juice than water.
Year:
2008
Link:
Study 6
Study type:
Randomised non-blinded crossover trial
Dose
250mL of beetroot juice (containing 5.5 mmol of nitrate) or placebo (250mL of water)
Participants:
9 healthy men and women.
Duration:
Acute (less than 24 hours)
Results:
Supplementation with beetroot juice (dietary nitrate) was associated with a 5.4 mmHg reduction in systolic blood pressure. The authors suggested that a dietary nitrate approach to cardiovascular disease may have therapeutic use.
Year:
2010
Link:
Study 7
Study type:
Randomised, double-blind, crossover trial
Dose
2 x 70 mL/day of organic beetroot juice (each containing 4.8 mmol of nitrate). The placebo consumed nitrate-depleted beetroot juice.
Participants:
12 healthy, normotensive, non-smoking, older adults (6 males and 6 female)
Duration:
2.5 days
Results:
2.5 days of dietary nitrate supplementation was associated with a four-fold increase in plasma nitrite concentration and significant reductions in resting blood pressure. More specifically, plasma nitrite increased to 418% of the placebo value, and blood pressure decreased by 5/3 mmHg relative to the placebo (115/70 vs 120/73 mmHg). The authors suggested that nitrate supplementation could potentially reduce the risk of hypertension and cardiovascular disease in older adults.
Year:
2013
Link:
Study 8
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Dose:
Nitrate-rich beetroot juice (12.9 mmol of nitrate) or a placebo (nitrate-depleted beetroot juice (0.5 mmol of nitrate)
Participants:
20 men and women (mean age: 62.5) with uncontrolled hypertension
Duration:
7 days
Results:
Supplementation with beetroot juice (dietary nitrate) was associated with a significant reduction in systolic and diastolic blood pressure compared to the placebo. Beetroot juice supplementation was also significantly associated with increased plasma nitrite. Significant decreases in 24h (−8/−4 mmHg) and day blood pressure (−9/−4 mmHg) profiles were observed.
Year:
2018
Link:
Study 9
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Dose:
150 mL of nitrate-rich beetroot juice (10.5 mmol of nitrate) or a placebo (1 mmol nitrate) 2.25 hours prior to a 30-min treadmill walk
Participants:
13 younger (18–30) and 11 older (50–70) normotensive adults
Duration:
Acute (3.5 hours)
Results:
Supplementation with beetroot juice (dietary nitrate) was associated with a significant reduction in systolic blood pressure in both age groups and diastolic blood pressure in older adults. Beetroot juice was also associated with increased plasma nitrate and nitrite concentrations.
The authors concluded that acute supplementation with beetroot may reduce blood pressure.
Year:
2019
Link:
Study 10
Study type:
Randomised, placebo-controlled, single-blind crossover trial
Dose:
140 mL of beetroot juice (containing 7.58 millimoles of nitrate) or a placebo (163 ml of prune juice with less than 0.01 millimoles of nitrate)
Participants:
15 patients with chronic obstructive pulmonary disease (11 males and 3 females)
Duration:
48 hours
Results:
Supplementation with beetroot juice (dietary nitrate) was associated with a significant reduction in resting systolic blood pressure (-8 mmHg), end-of-exercise diastolic blood pressure (-5 mmHg) and a trend for a decrease in resting diastolic blood pressure (−3 mmHg). Beetroot juice was also associated with increased plasma nitrate (+938%) and nitrite (+379%) relative to the placebo.
The authors concluded that dietary nitrate supplementation may reduce blood pressure and elevate plasma nitrate and nitrite concentrations.
Year:
2015
Link:
Study 11
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Dose:
Nitrate-rich beetroot juice (500mg/8.1mmol of nitrate) or a placebo (nitrate-depleted beetroot juice with less than 0.08 mmol of nitrate)
Participants:
18 untreated hypertensives aged 44 on average.
Duration:
Acute (8 hours)
Results:
Supplementation with beetroot juice (dietary nitrate) was associated with a larger reduction in ambulatory systolic and diastolic blood pressure (-6.7/-5.2 mmHg) compared to the placebo (-0.8/-1.7 mmHg) after 8 hours.
Year:
2018
Link:
Study 12
Study type:
Randomised controlled trial
Dose:
∼5.76 mmol of nitrate in the form of a concentrated beetroot juice drink (55 mL), a non-concentrated beetroot juice drink (456 mL) and a solid beetroot flapjack (60 g). A drink containing soluble beetroot crystals (∼1.40 mmol of nitrate) and a control drink (70mL of deionised water) were also ingested.
Participants:
10 healthy males
Duration:
Acute (24 hours)
Results:
Beetroot juice (dietary nitrate) was associated with lower blood pressure and higher concentrations of nitric oxide metabolites. All nitrate-rich vehicles in the study were associated with elevated plasma, salivary and urinary nitric oxide metabolites compared with baseline and the control.
Year:
2018
Link:
Study 13
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Dose:
70mL of beetroot juice (400 mg of nitrate) or a placebo (nitrate-depleted beetroot juice)
Participants:
14 healthy males (aged 22 on average)
Duration:
15 days
Results:
Compared with the placebo, beetroot juice (dietary nitrate) was associated with reductions in systolic and diastolic blood pressure, mean arterial pressure and total peripheral resistance at rest and during exercise.
Beetroot juice was also associated with significant increases in baseline concentrations of plasma nitrate and nitrite compared to the placebo.
Year:
2015
Link:
Study 14
Study type:
Randomised, double-blind, placebo-controlled trial
Dose:
500 mL/day of beetroot juice (containing ∼6.2 mmol of nitrate) or a placebo (500 mL/day of nitrate-depleted beetroot juice)
Participants:
9 normotensive, physically active males
Duration:
6 days
Results:
Short-term supplementation with beetroot juice (dietary nitrate) was associated with significant increases in plasma nitrite (+105%) and reductions in systolic blood pressure (-5 mmHg) in normotensive young men consuming a normal, balanced diet. The placebo had no effect on systolic, diastolic or mean arterial blood pressure.
Year:
2011
Link:
Study 15
Study type:
Randomised double-blind crossover trial
Dose
24 mmol of potassium nitrate (1488mg of nitrate) in capsules or a placebo (24 mmol of potassium chloride)
Participants:
21 healthy men and women
Duration:
Acute (less than 24 hours)
Results:
Potassium nitrate supplementation was associated with substantial reductions in systolic and diastolic blood pressure over 24 hours, whereas a similar dose of potassium chloride did not alter blood pressure over the same time period. These findings suggest that the changes in blood pressure were not attributable to the potassium content. Instead, the changes were likely dependent on the endogenous conversion to nitrite and, thereupon, to nitric oxide: the changes in plasma nitrite correlated closely with reductions in blood pressure.
Kapil et al.’s findings also showed dose-dependent reductions in systolic blood pressure with incremental doses of inorganic nitrate (4mmol and 12mmol).
Year:
2010
Link:
Study 16
Study type:
Systematic review
Intervention under study:
Beetroot juice (dietary inorganic nitrate) supplementation
Studies reviewed:
11 randomised controlled trials published between 2008 and 2018
Results:
The review concluded that supplementation with beetroot juice may reduce blood pressure in different populations, probably through the nitrate-nitrite/nitric oxide pathway and secondary metabolites found in beetroot. The review also concluded that beetroot juice may significantly decrease the risk of suffering cardiovascular events, and the authors believe that beetroot juice should be promoted as a key component of a healthy lifestyle to control blood pressure in healthy and hypertensive individuals.
Year:
2018
Link:
Study 17
Study type:
Systematic review and meta-analysis
Intervention under study:
Dietary nitrate supplementation
Outcome under study:
Medium-term effects of dietary nitrate supplementation on systolic and diastolic blood pressure.
Studies included:
13 randomised clinical trials with 7 to 65 participants per study. Most of the trials were placebo-controlled (75%).
Results:
Overall, dietary nitrate supplementation for more than one week was associated with a significant decrease in systolic and diastolic blood pressure. The pooled effect for the two interventions showed a reduction in systolic blood pressure (-4.1 mmHg) and diastolic blood pressure (-2.0 mmHg).
Year:
2017
Link:
Study 18
Study type:
Systematic review and meta-analysis
Intervention under study:
Dietary nitrate supplementation. The intervention time ranged from 3 to 60 days with daily dosages of 70–250 mL of beetroot juice.
Outcome under study:
The role of dietary nitrate in lowering blood pressure in patients older than 18 with arterial hypertension ( > 130/80 mmHg).
Studies included:
7 single/double-blinded randomised controlled trials published between 2013 and 2020.
Results:
Inorganic nitrate derived from beetroot juice was associated with reductions in systolic blood pressure in patients with arterial hypertension. The authors concluded that dietary nitrate from beetroot juice may be an effective method to reduce the blood pressure of patients with arterial hypertension (in interventions up to 2 months duration).
Year:
2022
Link:
Study 19
Study type:
Systematic review and meta-analysis
Intervention under study:
Dietary inorganic nitrate/nitrite
Outcome under study:
Prevention and treatment of cardiovascular disease risk factors in humans
Studies included:
34 studies were included for qualitative synthesis, 23 of which were eligible for meta-analysis.
Results:
Inorganic nitrate intake was associated with significant reductions in resting blood pressure (systolic blood pressure: -4.80 mmHg; diastolic blood pressure: 1.74 mmHg).
Year:
2018
Link:
Study 20
Study type:
Randomised, double-blind, placebo-controlled trial
Dose
Beetroot juice with 6.5–7.3 mmol of nitrate. The placebo group received nitrate-depleted beetroot juice(<0.06 mmol nitrate)
Participants:
15 healthy, normotensive men and women aged 22 to 40
Duration:
Acute: 24 hours on two occasions (crossover)
Results:
Supplementation with beetroot juice containing inorganic nitrate was associated with lower aortic systolic blood pressure after 30 minutes (-5.2 mmHg).
Year:
2019
Link:
Study 21
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Dose:
70mL of beetroot juice (6.1 mmol nitrate). The placebo group consumed 70mL of nitrate-depleted beetroot juice.
Participants:
20 patients suffering from heart failure with preserved ejection fraction (aged 69 on average)
Duration:
First phase: acute (24 hours)
Second phase: 1 week
Results:
In both study phases, supplementation with nitrate-rich beetroot juice was associated with significant reductions in systolic blood pressure (resting) and increases in plasma nitrate and nitrite. After a single, acute dose of nitrate-rich beetroot juice, resting systolic blood pressure was significantly lower than the placebo (127 mmHg vs. 134 mmHg).
Compared to the placebo, plasma nitrite levels increased significantly after the nitrate-rich beetroot juice (38% after an acute dose and 129% after 1 week of daily doses).
The authors concluded that beetroot may significantly improve blood pressure in elderly patients with heart failure.
Year:
2016
Link:
Study 22
Study type:
Randomised placebo-controlled crossover trial
Dose
500 mL/day of beetroot juice (5.2 mmol of nitrate/day) and placebo (500 mL/day of juice). Participants also engaged in moderate-intensity exercise after 2.5 hours and on day 5 and day 15.
Participants:
8 healthy subjects (5 males and 3 females) with an average age of 29
Duration:
Acute (2.5 hours) and chronic (up to 15 days)
Results:
Nitrate-rich beetroot juice supplementation was associated with significantly lower systolic and diastolic blood pressure throughout the supplementation period (∼-4%). It was also associated with significantly elevated plasma nitrite concentration (+39% after 2.5 hours; +25% after 5 days; and +46% after 15 days).
The results indicate that dietary NO3− supplementation may acutely reduce blood pressure, and that this effect may be maintained for at least 15 days if supplementation is continued.
Year:
2010
Link:
Study 23
Study type:
Randomised unblinded crossover trial
Dose:
Participants were assigned either:
1. 200mL of beetroot juice (with ~800mg of nitrate) and 40 minutes of moderate-intensity aerobic exercise
2. 200mL of low-nitrate fruit soda and the same exercise
3. 200mL of water (insignificant nitrate) and no exercise.
Participants:
14 non-hypertensive obese males
Duration:
Acute (24 hours)
Results:
Compared to the control, supplementation with beetroot juice (dietary nitrate) was associated with a reduction in systolic ambulatory blood pressure (-5.3 mmHg) up to 6 hours after ingestion and moderate-intensity aerobic exercise. Beetroot juice supplementation was also associated with a significantly higher plasma nitric oxide concentration up to 1 hour after ingestion.
The authors concluded that inorganic nitrate may have important therapeutic applications to decrease the blood pressure response to exercise when individuals have an increased cardiovascular risk.
Year:
2019
Link:
Study 24
Study type:
Uncontrolled clinical trial
Intervention:
Beetroot juice concentrate and blackcurrant juice
Participants:
21 men and women
Duration:
3 weeks
Results:
Beetroot juice concentrate was associated with a ~7.3mmHg reduction in daily systolic blood pressure after 3 weeks. However, beetroot juice supplementation was not associated with significant changes in resting clinic blood pressure or 24h ambulatory blood pressure.
Year:
2016
Link:
Study 25
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Dose:
500 mL/day of either nitrate-rich beetroot juice (containing 5.1 mmol of nitrate/day) or placebo (a drink with negligible nitrate content
Participants:
7 men aged 19 to 38
Duration:
6 days
Results:
Overall, systolic and diastolic was significantly lower after 6 days of nitrate supplementation (-7/7 mmHg). Relative to the placebo, nitrate supplementation was associated with reductions in systolic blood pressure (-5 mmHg), diastolic blood pressure (-2 mmHg) and mean arterial pressure (-2 mmHg).
Year:
2010
Link:
Study 26
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Dose:
0.11 mmol of nitrate per kg of body weight (a body mass-normalised moderate dose of nitrate) via beetroot juice.
Participants:
11 patients with peripheral artery disease
Duration:
Acute (∼1 hour)
Results:
Compared to the placebo, dietary nitrate supplementation was associated with reductions in peripheral and central systolic blood pressure (−4.7 mmHg and −8.2 mmHg, respectively) and significant increases in serum nitrate/nitrite level.
The authors concuded that acute, body mass-normalised, moderate doses of dietary nitrate may improve blood pressure and nitric oxide bioavailability.
Year:
2021
Link:
Study 27
Study type:
Randomised crossover trial
Dose:
∼400mg of nitrate at lunch, provided through nitrate-rich vegetables or beetroot juice supplementation
Participants:
15 healthy men and women (aged 24 on average)
Duration:
1 week
Results:
Nitrate-rich vegetables and beetroot juice supplementation were associated with increases in plasma nitrate concentrations and reductions in mean systolic and diastolic blood pressure throughout both intervention periods (∼2.5 hours after lunch).
Year:
2020
Link:
Study 28
Study type:
Systematic review and meta-analysis
Intervention under study:
Dietary inorganic nitrate (with doses ranging from 150 to 1000mg over a treatment range from 7 to 168 days).
Outcome under study:
Effect of repeated administrations of inorganic nitrate on blood pressure and arterial stiffness.
Studies included:
47 randomised controlled trials, including 1101 participants (including healthy, overweight, hypertensive, diabetic and hypercholesterolemic individuals, and patients with heart failure and peripheral artery disease).
Results:
The results found that inorganic nitrate supplementation was associated with an overall beneficial effect on blood pressure. Repeated (≥ 3 days) administrations of inorganic nitrates were associated with reductions in peripheral and central blood pressure.
Year:
2020
Link:
Study 29
Study type:
Open-label randomised controlled trial
Dose:
70mL of beetroot concentrate (containing ~300 to 400mg of nitrate)
Participants:
21 overweight men and women between the ages of 55 and 70
Duration:
28 days
Results:
Supplementation with beetroot juice (dietary nitrate) was associated with a 10.2/3.1 mmHg reduction in blood pressure after 3 weeks of supplementation.
Year:
2015
Link:
Study 1
Study type:
Randomised double-blind cross-over trial
Intervention & outcome:
Researchers studied the effects of acute beetroot ingestion on cycling performance in normobaric hypoxic conditions. The performance trials consisted of submaximal steady-state exercise for 15 minutes at 60% maximum work rate and, after a 5 minute break, a 16.1 km time trial at simulated altitude. During the time trial, participants were encouraged to complete the 16.1 km in the shortest time possible. The heart rate, peripheral oxygen saturation and respiratory variables were continuously monitored throughout each trial.
Dose:
The performance trials were preceded by ingestion of either 70mL of nitrate-rich beetroot juice (~5 mmol of nitrate) or a placebo (nitrate-depleted beetroot juice) 3h before exercise.
Participants:
9 competitive amateur male cyclists (aged 28 on average)
Duration:
Acute
Results:
A single dose of beetroot juice was associated with reduced oxygen cost of steady-state exercise and enhanced 16.1-km time trial performance at simulated altitude. 8 of the 9 participants were quicker during the beetroot trial than during the placebo trial. Beetroot juice was associated with a 2.9% performance improvement compared with baseline (1716 s at baseline; 1664 s after beetroot) with a medium effect size. Performance after beetroot ingestion was significantly improved compared with the placebo (1702 s after placebo). Performance was not different between baseline and placebo trials.
Year:
2014
Link:
Study 2
Study type:
Systematic review
Intervention under study:
Dietary nitrate supplementation
Outcome under study:
Muscular power, velocity of contraction and muscular endurance during weightlifting in healthy adults
Studies included:
4
Results:
2 of the 4 studies included in the review indicate that nitrate supplementation may increase aspects of upper body exercise performance (i.e. bench press): there were associations with increases in mean power, velocity of contraction and number of repetitions to failure. Another study observed an increase in the number of repetitions to failure during back squats.
Year:
2020
Link:
Products Containing Beetroot
Beetroot Tablets 3000mg
Just £0.62 per day

L-CITRULLINE SCIENTIFIC STUDIES
We have summarised some of the most interesting scientific studies on L-citrulline.
It is important to note that a decrease between 5 and 12 mmHg of systolic blood pressure and between 5 and 6 mmHg of diastolic blood pressure is associated with a 14–38% risk reduction in stroke and a 21% risk reduction of death due to coronary disease.
Study 1
Study type:
Randomised controlled trial
Participants:
12 young adults with normal blood pressure
Dose:
3g of citrulline tablets per day
Duration:
1 week
Results:
Citrulline consumption was associated with a 6% reduction in systolic blood pressure and a 14% reduction in diastolic blood pressure.
Year:
2016
Link:
Study 2
Study type:
Randomised controlled trial
Participants:
41 obese postmenopausal women
Duration:
8 weeks
Results:
L-citrulline supplementation was associated with significant reductions in blood pressure.
Year:
2015
Link:
Study 3
Study type:
Randomised controlled trial
Dose:
3g per day
Participants:
Heart failure patients
Duration:
2 months
Results:
L-citrulline supplementation was associated with significant reductions in systolic and diastolic arterial blood pressure.
Year:
2015
Link:
Study 4
Study type:
Systematic review
Intervention under study:
Oral supplementation with L-citrulline
Studies reviewed:
8 randomised controlled trials
Results:
The results suggest that L-citrulline supplementation may reduce systolic blood pressure (-4 mmHg). A significant reduction in diastolic blood pressure was observed only in the studies that used doses greater than 6 g of L-citrulline per day.
Year:
2019
Link:
Study 1
Study type:
Randomised controlled trial
Dose:
6 g L-citrulline-malate 2 h prior exercise
Participants:
17 male pre-professional cyclists
Duration:
Acute (before and after a 137-km cycling race)
Results:
After the cycling race, growth hormone levels were higher among participants taking L-citrulline malate than among participants in the control group. L-citrulline supplementation was also associated with higher levels of arginine-derived metabolites such as nitrite, creatinine, ornithine and urea.
Year:
2010
Link:
Study 2
Study type:
Randomised placebo-controlled crossover trial
Dose:
2.4g of L-citrulline for 7 days and 2.4 g of L-citrulline 1h before a 4-km cycling time trial on day 8.
Participants:
22 athletically-trained males
Duration:
8 days
Results:
Compared to the placebo, L-citrulline supplementation was associated with a 1.5% reduction in the time taken to complete a 4-km cycling time trial. It was also associated with significant increases in plasma L-arginine levels.
Year:
2016
Link:
Study 3
Study type:
Systematic review
Intervention under study:
L-citrulline supplementation before exercise
Studies reviewed:
13 studies comprising 206 participants
Results:
The results show a correlation between citrulline supplements and significant reductions in the rate of perceived exhaustion during physical activity and muscle soreness 24h and 48h after exercise.
The results suggest that athletes may benefit from ingesting either L-citrulline alone or 1h before exercise to resist fatigue or relieve muscle soreness.
Year:
2021
Link:
VITAMIN D SCIENTIFIC STUDIES
There is a large amount of evidence to suggest that adequate levels of vitamin D in the blood can have protective effects against COVID-19, dementia, diabetes and autoimmune disease. We have summarised some of the most interesting scientific studies.
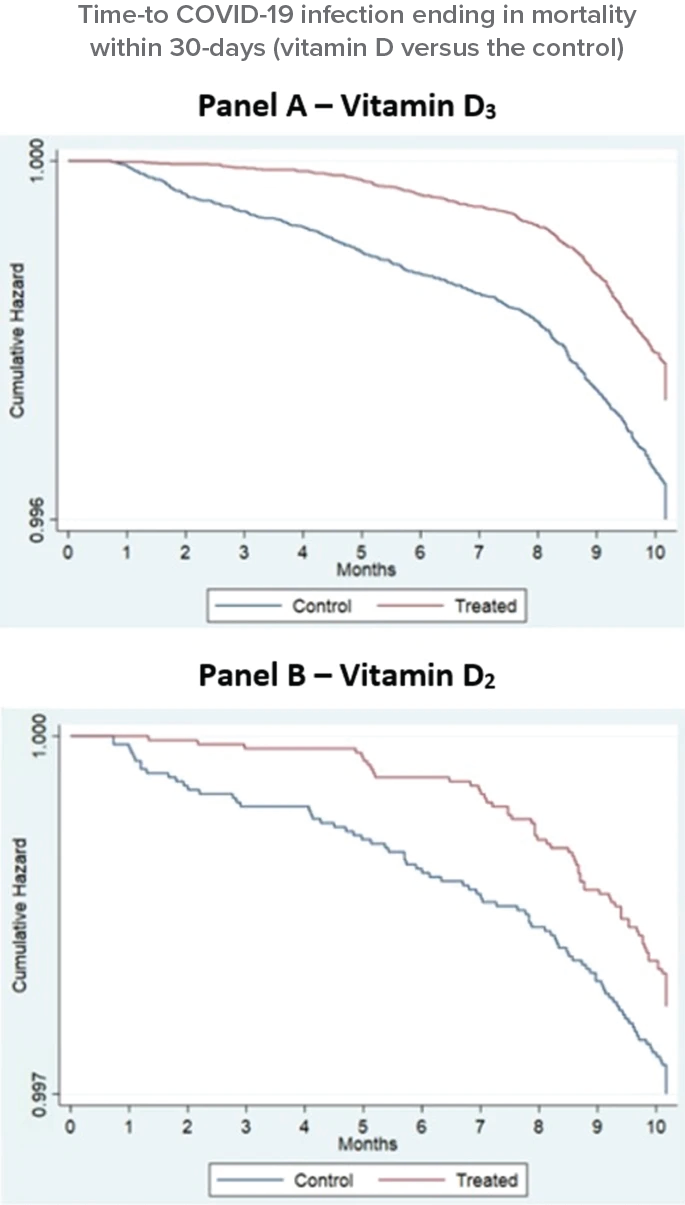
Study 1
Study type:
Retrospective cohort study. This type of observational study in which a group of individuals with a common exposure are compared to another group of individuals using historical records.
Purpose:
To investigate whether Vitamin D treatment can reduce the associated risk of COVID-19 infection.
Subjects:
- 220,265 American veterans supplemented with vitamin D3 (cholecalciferol or calcifediol)
- 34,710 supplemented with vitamin D2 (ergocalciferol or doxercalciferol)
- 407,860 untreated patients (control)
Duration:
January 1st 2019 to December 12th 2020 (before and during the pandemic)
Doses:
Dosage options included 20 IU, 40 IU, 100 IU, 125 IU, 200 IU, 250 IU, 400 IU, 500 IU, 800 IU, 1000 IU, 2000 IU, 5000 IU, 8000 IU, and 50,000 IU.
Source of data:
Veterans Administration Corporate Data Warehouse (CDW) electronic health records between January 1, 2019, and December 31, 2020
Results:
In US veterans, Vitamin D supplementation during the pandemic was associated with a significant 20% and 28% reduction in COVID-19 infection rates for vitamin D3 and vitamin D2, respectively.
Vitamin D3 was associated with a significant 33% decrease in mortality within 30-days of COVID-19 infection and 25% lower with Vitamin D2.
Veterans receiving higher cumulative doses and higher average daily doses of Vitamin D had a greater associated reduction in COVID-19 infection rates than veterans receiving lower doses. The 50,000 IU dosage may be especially beneficial.
Based on these results, there would have been approximately 4 million fewer COVID-19 cases and 116,000 deaths avoided in 2020. These values were calculated by applying the estimated 20% average reduction in infection and 33% reduction in death after infection to a total of 19,860,000 cases and 351,999 deaths through 2020.
The COVID-19 rates were 2.66% among participants treated with vitamin D3 and 3.30% among the untreated participants, The rates of COVID-19 infection followed by death within 30 days were 0.23% among participants treated with vitamin D3 and 0.35% among untreated participants.
Conclusion:
Vitamin D3 could be a helpful tool for reducing the spread of COVID-19 infection and related deaths, particularly as a large proportion of the UK and US population have suboptimal blood levels of vitamin D.
Year:
2022 (November 12th)
Link:
Study 2
Study type:
Meta-analysis and trial sequential analysis
Authors:
Leading Italian doctors and scientists
Purpose:
To explain the strength of the association between the protective role of vitamin D supplementation and the risk of death and admission to intensive care units (ICUs) in patients with COVID-19.
Studies analysed:
5 randomised clinical trials that had been published before September 2022
Doses analysed:
5000 IU; 10,000 IU; 21,280 IU; 21,260 IU; 200,000 IU
Results:
The study found a conclusive association between vitamin D supplementation and a decreased risk of death (0.49) and ICU admission (0.28) among patients with COVID-19. This means that vitamin D was associated with 51% better protection against death and 72% better protection against ICU administration.
Using "trial sequential analysis", a method used to determine if there is enough evidence to draw a conclusion or if more data is needed, the study determined that the pooled data conclusively shows a positive association between vitamin D supplementation and reduced risk of death and ICU admission in COVID-19 patients.
Year:
2023 (January 16th)
Link:
Study 3
Study type:
Randomised clinical trial
Purpose:
To determine the effects of 5000 IU versus 1000 IU of daily vitamin D3 supplementation on the recovery of symptoms among mild to moderate COVID-19 patients with sub-optimal vitamin D levels.
Dose:
5000 IU or 1000 IU of vitamin D. The study used 1000 IU as the control because it would be unethical to give a placebo to individuals with suboptimal vitamin D levels. 1000 IU is regarded as a standard control.
Participants:
69 men and women
Duration:
90 days
Results:
Among patients with sub-optimal vitamin D status and mild to moderate COVID-19 symptoms, 5000 IU of daily oral vitamin D3 supplementation for 2 weeks was associated with a significantly shorter time to recovery for coughs and loss of taste. 5000 IU of vitamin D resulted in a significant increase in vitamin D levels in the blood, whilst 1000 IU of vitamin D did not.
Year:
2021
Link:
Study 4
Study type:
Randomised clinical trial
Purpose:
To evaluate the effect of vitamin D3 treatment on intensive care unit admissions and death rates among Spanish patients hospitalised with COVID-19.
Dose:
21,280 IU (0.532 mg) of oral vitamin D3 (calcifediol) on days 1,3 and 7 and then weekly until discharge or ICU admission. The control group received no vitamin D. All patients also received standard treatments for COVID.
Participants:
76 Spanish patients hospitalised with COVID-19
Duration:
4 weeks
Results:
Vitamin D administration was associated with significant reductions in the need for ICU treatment of patients requiring hospitalisation due to proven COVID-19. Vitamin D was also associated with reductions in the severity of COVID-19.
Year:
2020
Link:
Study 5
Study type:
Randomised clinical trial (single-blind)
Purpose:
To evaluate the effect of vitamin D3 on the immune response against COVID-19 in individuals with severe COVID-19 symptoms.
Dose:
10.000 IU of daily vitamin D3 or a control (2000 IU of vitamin D3)
Participants:
85 patients hospitalised with COVID-19
Duration:
8 to 29 days
Results:
Vitamin D3 administration was associated with shorter hospital stays and improvements in the inflammatory and cytotoxic response against COVID-infected cells.
Year:
2022
Link:
Study 6
Study type:
Clinical trial (non-randomised)
Purpose:
To evaluate the effect of vitamin D3 treatment on COVID-19–related outcomes
Dose:
21.620 IU on day 1 (532 mcg) and 10.810 IU on days 3, 7, 15, and 30 (266 mcg)
Participants:
838 patients with COVID-19
Study duration:
March to May 2020
Results:
In patients hospitalised with COVID-19, treatment with vitamin D3 was associated with significantly reduced ICU admission and death.
Year:
Published in 2021
Link:
Study 1
Study type:
Systematic review & meta-analysis
Purpose:
To evaluate whether administration of vitamin D decreases risk for diabetes among people with prediabetes.
Studies analysed:
3 randomised clinical trials
Doses analysed:
20 000 IU (500 mcg) weekly; cholecalciferol, 4000 IU (100 mcg) daily; or eldecalcitol, 0.75 mcg daily, versus matching placebos.
Results:
Vitamin D reduced risk for diabetes by 15% in adjusted analyses, with a 3-year absolute risk reduction of 3.3%.
Among participants assigned to the vitamin D group who maintained a blood level of at least 50 ng/mL compared with 20 to 29 ng/mL, vitamin D3 supplementation was associated with a reduced risk for diabetes by 76%, with a 3-year absolute risk reduction of 18.1%.
Conclusion:
In adults with prediabetes, vitamin D may be effective at decreasing risk for diabetes.
Year:
2023
Link:
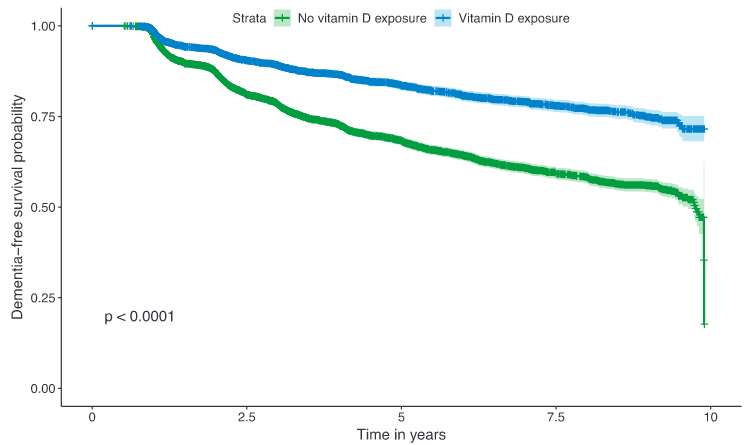
Study 1
Study type:
Cohort study (a group of individuals were observed over a long period of time to evaluate the effects of vitamin D supplementation)
Purpose:
To evaluate the effects of Vitamin D on the incidence of dementia.
Number of participants:
12,388 participants with average age of 71
Study duration:
10 years
Results:
Vitamin D exposure was associated with 40% lower dementia rates versus no exposure to vitamin D. Participants taking vitamin D had less mild cognitive impairment and less depression.
2,696 of the 12,388 participants developed dementia. Among these 2,696 participants, 2,017 (74.8%) had no exposure to vitamin D. Thus, the majority of participants who developed dementia did not take any vitamin D. The researchers did not find any difference in effects between vitamin D3 and D2.
Year:
2023
Link:
Study 2
Study type:
Meta-analysis
Purpose:
To estimate the association between vitamin D deficiency and risk of developing Alzheimer’s disease and dementia.
Studies analysed:
5 observational studies
Results:
The meta-analysis showed that subjects with deficient vitamin D status (with blood levels less than 20 ng/mL) were at increased risk of developing Alzheimer’s disease by 21% compared with those with vitamin D levels greater than 50 nmol/L.
Conclusion:
Vitamin D status may be associated with increased risk of developing Alzheimer’s disease and dementia.
Year:
2015
Link:
Study 1
Study type:
Randomised clinical trial (double-blind and placebo-controlled)
Purpose:
To investigate whether vitamin D and marine-derived long chain omega 3 fatty acids reduce the risk of autoimmune disease.
Dose:
- 2000 IU (50 mcg) per day of vitamin D per day or matched placebo,
- 1000 mg/day omega 3 fatty acids or matched placebo.
Participants:
There were 25 871 participants (12 786 men ≥ 50 years old and 13 085 women ≥ 55 years old).
Duration:
~5 years
Results:
Among the participants who received vitamin D, 123 individuals developed an autoimmune disease compared to 155 individuals in the placebo group (statistically significant). Although omega 3 fatty acid supplementation was associated with a lower rate of autoimmune disease, the results were not statistically significant.
Conclusion:
Vitamin D was associated with a 22% reduction in autoimmune disease (based on a hazard ratio of 0.78).
Year:
2022
Link:
Products Containing Vitamin D3
Vitamin D3 and K2 MK7 Tablets
Just £0.08 per day

NMN Scientific Studies
Several studies have investigated the effects of NMN. They have indicated that a dose of 500mg of NMN is the most effective without any adverse side effects. We have summarised the most interesting results from rodent studies and clinical trials.
Study 1
Study type:
Randomised, double-blind, placebo-controlled, parallel-group trial
Purpose:
To evaluate whether chronic NMN supplementation elevates blood NAD+ levels and affects physiological dysfunctions in healthy older participants. NAD+ is a critical molecule that supports energy production, DNA repair, and cellular communication.
NMN dose:
250 mg/day or a placebo
Duration:
6 or 12 weeks
Participants:
42 men aged over 65 years
Results:
Long-term NMN supplementation was well tolerated and caused no significant adverse effects. Blood analyses revealed significant increases in NAD+ and its related metabolites concentrations, which play crucial roles in processes associated with energy production and tissue repair Research has found that NAD+ levels naturally decline with age, and an increase in NAD+ may enhance energy metabolism, support cell and tissue repair, and contribute to overall well-being.
The researchers observed significant improvements in muscle performance like grip strength and walking speed. It is worth noting that reductions in strength are a clinical indicator of ageing.
The authors concluded that long-term NMN supplementation may be used to boost NAD+ for preventing age-related muscle dysfunctions in humans.
Year:
2022
Link:
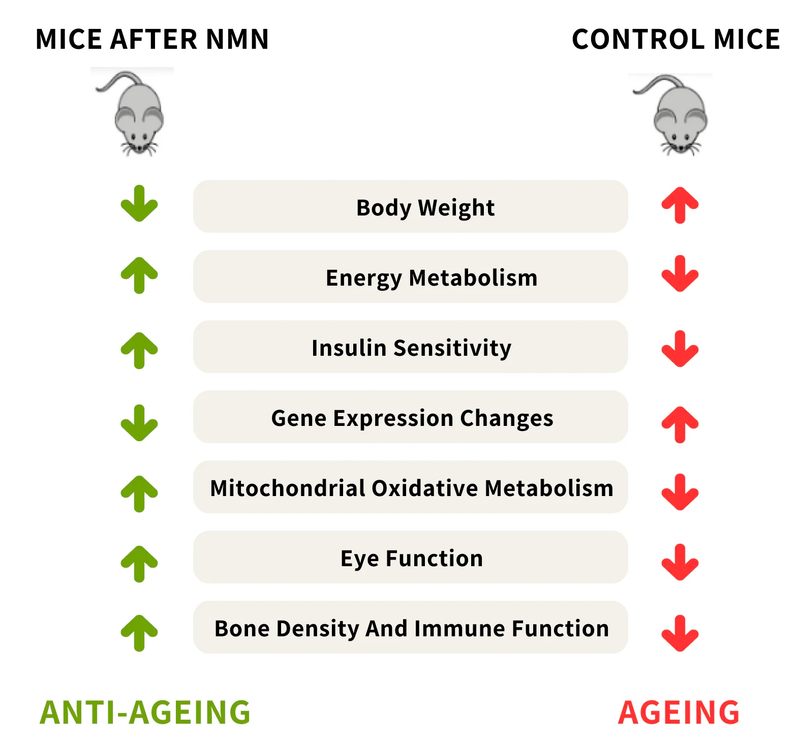
Study 2
Study type:
Animal study (mice)
Purpose:
To investigate the long-term effects of NMN administration on age-associated physiological decline in mice.
Age-related complication:
Age-associated physiological decline
NMN dose:
100 and 300 mg/kg for 12 months
Duration:
12 months
Results:
The study found that orally administered NMN was quickly absorbed, efficiently transported into blood circulation, and immediately converted to NAD+ in major metabolic tissues (tissues are groups of similar cells that work together to perform a specific function in the body). NAD+ is a crucial coenzyme involved in various cellular processes, including energy production, DNA repair, and cell signalling.
In addition, the study found that NMN suppressed age-associated body weight gain and enhanced energy metabolism, improved insulin sensitivity, eye function, and other features with no toxic effects. NMN also prevented age-associated gene expression changes. This means that NMN may have the ability to counteract or prevent the alterations in gene activity that typically occur as a result of ageing.
These effects indicate that NMN (and other NAD+ intermediates) may be an effective anti-ageing intervention in humans, although clinical studies are required.
Year
2016
Link:
Study 3
Study type:
Animal study (aged mice)
Purpose:
To investigate the effects of NMN supplementation on blood vessel dysfunction (a condition where the inner lining of blood vessels does not function properly) and oxidative stress (an imbalance between harmful molecules called free radicals and antioxidants in the body), which are both associated with ageing and contribute to an increased risk of cardiovascular diseases, impaired cellular function, chronic inflammation, and accelerated ageing.
Age-related complication:
Age-associated vascular dysfunction and oxidative stress
NMN dose:
300 mg/kg body weight/day in drinking water or a control (just drinking water)
Duration:
8 weeks
Results:
8 weeks of NMN supplementation restored a marker of arterial sirtuin activity. Sirtuins are proteins that are known to have protective effects on blood vessels and promote healthy ageing. NMN also improved age-associated blood vessel dysfunction and artery stiffening. Artery stiffening refers to the loss of flexibility and increased rigidity of blood vessel walls, which can lead to reduced blood flow, elevated blood pressure, and an increased risk of cardiovascular diseases.
These improvements were associated with restored nitric oxide, a molecule that plays a crucial role in regulating blood vessel function, as well as the either complete or partial normalisation of structural proteins in the arterial wall and a reduction of vascular oxidative stress, which is important for maintaining the health and function of blood vessels and reducing the risk of related diseases.
The results provide evidence that oral supplementation with NMN may restore SIRT1 activity (a sirtuin gene) and potentially combat or reverse age-related arterial dysfunction by decreasing oxidative stress.
Year
2016
Link:
Study 4
Study type:
Animal study (aged mice)
Age-related complication:
Age-associated decline in DNA repair
NMN dose:
500 mg/kg body weight/day
Duration:
7 days
Results:
NMN treatment reduced DNA damage and protected against changes in haemoglobin, a protein found in red blood cells that plays a crucial role in transporting oxygen from the lungs to the body's tissues and organs. NMN also protected against changes in white blood cell count (including lymphocytes). Reducing DNA damage and maintaining stable haemoglobin and white blood cell levels contribute to better cellular health, improved oxygen transport, and a well-functioning immune system. These factors collectively support overall health and reduce the risk of various age-related diseases and health complications.
Year
2017
Link:
Study 5
Study type:
Animal study (rats)
Purpose:
To compare NMN with nicotinamide (vitamin B3) as a superior precursor of NAD in mice. NAD plays a vital role in supporting mitochondrial function for cellular energy production, but its levels decrease with age, affecting energy production, DNA repair, and essential processes. Maintaining healthy NAD levels may help mitigate some of the effects of ageing.
NMN dose:
45 μmol//kg body weight injected intraperitoneally (single dose)
Results:
Compared to nicotinamide, NMN resulted in a higher increase in NAD+, activated a higher response of SIRT1 (a sirtuin gene linked to healthy ageing) and had greater anti-aging activity.
These findings may indicate enhanced energy production, improved DNA repair, better cellular health, and potentially slowing down of the ageing process.
Year
2016
Link:
Study 6
Study type:
Animal study (aged mice)
Purpose:
In mice supplemented with NMN, researchers assessed the role of NAD+ (a critical molecule for cellular functions) and SIRT1 (a protein known as a sirtuin, which is involved in regulating cellular processes related to ageing and relies on NAD for its activity) in acute kidney injury susceptibility. NMN is known as the building block for NAD.
Age-related complication:
Age-associated susceptibility to acute kidney injury
NMN dose:
500 mg/kg body weight/day (intraperitoneal)
Duration:
4 days
Results:
NMN supplementation increased NAD+ levels (an important molecule for energy production, DNA repair, and cell signalling) in aged kidneys. This increase in NAD+ levels was also associated with increased activity of SIRT1 (a protective protein), and protected aged kidneys from both ischemia–reperfusion (reduced blood flow) and chemotherapy-related kidney injuries.
Year
2017
Link:
Study 7
Study type:
Animal study (aged mice)
Purpose:
To explore the potential of NMN in restoring capillary density and improving endurance in old mice. Capillaries are the smallest blood vessels in the body. Capillary density is important for the exchange of oxygen, nutrients, and waste products between the blood and tissues.
Age-related complication:
Blood vessel ageing
NMN dose:
400 mg/kg body weight/day (in drinking water)
Duration:
2 months
Results:
NMN improved blood flow by restoring the capillary density (the number of tiny blood vessels) of elderly mice to youthful levels. Capillary density is important for various physiological processes, including tissue growth, repair, and metabolism. NMN also improved the treadmill endurance of old mice towards youthful levels.
Year
2018
Link:
Study 1
Study type:
Randomised, multicentre, double-blind, placebo-controlled, parallel group, dose-dependent trial
Purpose:
The primary objective was to evaluate blood concentrations of nicotinamide adenine dinucleotide (NAD) with different doses of NMN. NAD is a vital molecule that acts like a helper in the body's cells, participating in important processes like turning food into energy and helping to repair damaged DNA. The secondary objectives were to assess the safety, tolerability and clinical efficacy of NMN supplementation.
Measurement methods:
Clinical efficacy was evaluated by conducting a subjective general health assessment (survey) and measuring NAD blood concentration, physical performance (six-minute walking test), blood biological age (Aging.Ai 3.0 calculator) and the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR). The HOMA-IR calculation involves analysing fasting blood glucose and insulin levels to provide an estimate of insulin resistance.
NMN dose:
300, 600 or 900 mg/day, or a placebo
Duration:
60 days
Participants:
80 middle-aged healthy adults
Results:
Compared to the placebo, the blood concentration of NAD significantly improved as the NMN dose increased (dose-dependent). An increase in NAD+ may enhance energy metabolism, support cell and tissue repair, and contribute to overall well-being. Research has found that NAD+ levels naturally decline with age. The most beneficial effects in the study, particularly on blood NAD levels and physical performance, were achieved with a daily oral intake of 600 mg. Oral administration of NMN up to 900 mg/day for 60 days was safe and well tolerated. The 900 mg/day oral dose did not have significantly better efficacy than the 600 mg/day dose.
In addition, the study found an association between NMN supplementation and improvements in physical endurance and general health conditions, as evidenced by significant improvements in measures such as the six-minute walking test, blood biological age, and general health survey when compared to a placebo. Overall, these findings suggest that NMN supplementation may improve health and physical performance.
Year:
2022
Link:
Study 2
Study type:
Randomised, double-blind, placebo-controlled, parallel-group trial
Purpose:
To investigate the influence of 12-week NMN supplementation on biochemical and metabolic health parameters.
NMN dose:
250 mg/day (2 x 125 mg/day) or a placebo
Duration:
12 weeks
Participants:
36 healthy, middle-aged men and women
Results:
The researchers observed that serum nicotinamide (NAD+) levels were significantly higher in the NMN intake group than in the placebo group. Nicotinamide is a form of vitamin B3 and a building block for NAD+, which plays a critical role in cellular energy production and other cellular processes. Low levels of nicotinamide in the blood may indicate a vitamin B3 deficiency, potentially impacting various aspects of health, as NAD+ is involved in many vital processes within the cells.
NMN supplementation showed potential in reducing arterial stiffness as indicated by a tendency of pulse wave velocity values, a measure of arterial stiffness, to decrease in the NMN group. Stiff arteries hinder blood flow, which can increase the risk of heart-related problems. However, no significant difference was found between the NMN and placebo groups.
Long-term NMN supplementation at 250 mg/day was well tolerated and did not cause adverse events. NMN safely and effectively elevated NAD+ metabolism in healthy middle-aged adults.
Year:
2023
Link:
Study 1
Study type:
Randomised double-blind placebo-controlled trial
Purpose:
To investigate the effect of NMN supplementation on metabolic function in postmenopausal women with prediabetes who are overweight or obese
NMN dose:
250 mg/day or placebo
Duration:
10 weeks
Participants:
25 postmenopausal women with prediabetes
Results:
The study found an association between NMN supplementation and increased muscle insulin signalling, which involves the phosphorylation (activation) of certain proteins such as protein kinase AKT and the targeting of rapamycin (mTOR), compared to the placebo group. These proteins work together and are crucial for maintaining proper blood sugar levels and ensuring the muscles have the energy they need to function effectively.
In addition, NMN supplementation was associated with increased muscle insulin sensitivity, indicating that the muscles became more responsive to the effects of insulin, a crucial factor for managing blood sugar levels and overall metabolic well-being. This improvement was measured by how quickly glucose was removed per unit of fat-free mass.
Year:
2021
Link:
Study 2
Study type:
Animal study (age-induced and diet-induced diabetic mice)
Purpose:
To investigate whether NMN could help treat diabetes caused by diet and ageing in mice.
Age-related complication:
Age- and diet-induced diabetes
NMN dose:
500 mg/kg body weight/day intraperitoneally for 7–10 days
Results:
Researchers observed several positive effects of NMN in mice with type 2 diabetes. Firstly, NMN improved glucose and lipid metabolism, specifically enhancing insulin secretion, insulin sensitivity, lipid profile, hepatic insulin sensitivity, and glucose tolerance. Improvements in these factors collectively contribute to maintaining healthy blood sugar levels, efficient energy metabolism, and overall cardiovascular health. When these aspects are functioning optimally, the risk of chronic diseases such as type 2 diabetes and cardiovascular diseases is lowered.
Secondly, NMN restored NAD+ levels (a molecule involved in energy metabolism) in the liver and body fat. Elevated NAD+ levels are generally associated with improved cellular health, enhanced metabolism, and potential benefits for longevity and overall well-being.
Thirdly, NMN normalised the inflammatory response, circadian rhythm (the body's internal clock that regulates sleep and other functions) and oxidative stress (cell damage caused by harmful molecules) affected by high-fat diets and ageing. These improvements were partly attributed to increased NAD+ levels and the activation of SIRT1, a protein associated with metabolic regulation and longevity.
The results provide evidence that promoting NAD+ biosynthesis by administering NMN may be an effective intervention to treat the pathophysiology of diet- and age-induced type 2 diabetes.
Year
2011
Link:
Study 1
Study type:
Animal (rats) and cellular study
Purpose:
To study the impact of NMN on beta-amyloid oligomer-induced neuronal death and cognitive impairment. These oligomers are considered to be toxic and are thought to play a significant role in the development and progression of Alzheimer's disease.
Age-related complication:
Alzheimer's disease
NMN dose:
500 mg/kg body weight/day
Duration:
10 days
Results:
NMN improved cognitive function, neuron survival and energy metabolism. These improvements potentially slow down the progression of cognitive decline. NMN restored levels of NAD+ (a key player in energy metabolism, DNA repair, and cell signalling) and ATP (the cell's primary energy currency) and eliminated the accumulation of reactive oxygen species (ROS), which are harmful molecules that can cause damage to cells and DNA This restoration of NAD+ and ATP levels and reduction in ROS accumulation is beneficial because it can potentially support better cellular energy production, protect neurons from oxidative stress, and contribute to the overall health of brain cells. This is particularly important in the context of Alzheimer's disease, where cellular dysfunction and oxidative damage contribute to the disease's progression.
Year
2016
Link:
Study 2
Study type:
Animal study (a mice model of Alzheimer’s disease)
Purpose:
To investigate the effect of NMN on brain mitochondrial impairments. Mitochondria are the energy-producing "powerhouses" of our cells.
Age-related complication under study:
Alzheimer’s disease
NMN dose:
100 mg/kg body weight subcutaneously every other day for 28 days
Duration:
28 days
Results:
Researchers observed improvements in the functioning of mitochondria, the energy-producing “powerhouses” of cells. In Alzheimer’s disease, there are known issues with the functioning of mitochondria. There was also a reduction in mutant amyloid precursor proteins, which may potentially slow down or prevent the progression of Alzheimer's disease.
NMN treatment also increased the levels of SIRT1 and CD38, which are proteins that play important roles in cellular processes that normally decline with age.
Year
2015
Link:
Study 3
Study type:
Animal study (mice)
Purpose:
To investigate the effects of NMN and its underlying mechanisms in mice with Alzheimer's disease.
Age-related complication:
Alzheimer’s disease
NMN dose:
100 mg/kg body weight/every other day (subcutaneously)
Duration:
28 days
Results:
NMN substantially improved cognitive impairments, neuroinflammation, beta-amyloid pathology and synaptic loss. Both beta-amyloid pathology (plaques) and synaptic loss are key features of Alzheimer's disease and are associated with cognitive decline and memory problems characteristic of the condition.
Year
2017
Link:
Study 4
Study type:
Animal study (aged rats)
Purpose:
To investigate the effect of NMN on cognitive outcomes in aged rats.
Age-related complication:
Cognitive impairment due to ageing
NMN dose:
100 mg/kg body weight/ every other day
Duration:
28 days
Results:
NMN had neuroprotective effects, reduced cognitive decline and helped improve age-associated memory and learning impairments.
Year
2019
Link:
Study 1
Study type:
Randomised double-blind placebo-controlled trial
NMN dose:
There were 3 different dosage groups and a placebo group:
- 300 mg/day (low dosage group)
- 600 mg/day (medium dosage group)
- 1200 mg/day (high dosage group)
Each group consisted of 10 male participants and 2 female participants.
Additional interventions:
All participants actively trained during the study period by adhering to an exercise program: 6 weeks of aerobic exercise (running and cycling), with 5–6 sessions per week lasting 40–60 min each.
Participants:
48 young and middle-aged recreational runners
Duration:
6 weeks
Results:
The researchers observed significant improvements in aerobic capacity (a measure of the body's ability to take in, transport, and utilise oxygen during sustained physical activity) and the endurance of amateur runners during exercise training after NMN supplementation, when compared to the placebo group. These improvements were dose dependent, meaning that the higher the NMN dose, the greater the effect on aerobic capacity and endurance. The authors attribute this improvement to enhanced oxygen utilisation in skeletal muscles. The moderate and higher doses were associated with greater improvements in aerobic capacity than the lower dose.
Year:
2021
Link:
Study 2
Study type:
Randomised double-blind placebo-controlled trial
Purpose:
To investigate the effects of the time-dependent intake of nicotinamide mononucleotide (NMN) on sleep quality, fatigue, and physical performance in older adults.
NMN dosage:
250 mg or a placebo either in the morning or in the evening to compare the effect of intake at different times.
Duration:
12 weeks
Participants:
108
Results:
The participants in the study reported reduced drowsiness after NMN supplementation. The study also revealed an association between NMN intake in the afternoon and significant improvements in lower body strength, balance, and functional mobility (the ability to move and perform daily tasks without difficulty). Overall, the authors concluded that NMN may have the potential to improve fatigue and prevent the loss of physical performance.
Year:
2022
Link:
https://doi.org/10.3390/nu14040755
Study 1
Study type:
Randomised, double blind, parallel-group trial
Purpose:
To investigate the safety of orally administered NMN and its efficacy to increase NAD+ levels.
NMN dose:
250 mg/day or a placebo
Duration:
12 weeks
Participants:
30 healthy subjects
Results:
Oral supplementation of NMN for 12 weeks caused no abnormalities in physiological and laboratory tests, and no obvious adverse effects were observed. NAD+ levels in whole blood were significantly increased after NMN administration. NAD+ plays a vital role in multiple cellular processes, and an increase in its levels suggests that the body has additional support for these vital processes, potentially resulting in favourable impacts on overall health and well-being. The authors concluded that oral administration of NMN may be a safe and practical strategy to boost NAD+ levels in humans.
Year:
2022
Link:
Study 2
Study type:
Randomised, double-blind, placebo-controlled, parallel-group trial
Purpose:
To evaluate the safety of 1250mg of β-NMN administered orally once daily.
NMN dose:
1250 mg/day or a placebo
Duration:
4 weeks
Participants:
31 healthy adult men and women aged 20-65
Results:
The researchers observed that oral administration of 1250 mg of NMN once daily for 4 weeks did not result in any severe adverse events during the study period. The findings suggest that NMN is safe and well-tolerated in healthy adults.
Year:
2022
Link:
Study 3
Study type:
Uncontrolled exploratory trial
Purpose:
To verify the safety of NMN administered through human veins.
NMN dose:
300 mg dissolved in 100mL of salt solution
Duration:
12 weeks
Participants:
5 males and 5 females (aged 20-70)
Results:
The study found an association between intravenous NMN administration and increased blood NAD+, a vital molecule that helps keep proper cell functioning and supports various aspects of overall health, without damaging blood cells.
Researchers also observed that NMN did not affect the participants’ heart's electrical activity, pulse, or blood pressure. It also did not impact metabolic markers in the liver, heart, pancreas, and kidneys. Additionally, the researchers noted a reduction in blood triglyceride (fat) levels after NMN administration. Elevated triglyceride levels have been linked to an increased risk of heart disease and other health issues. Intravenous administration normally carries a higher risk of damaging major organs compared to oral administration. This is because nutrients and drugs administered intravenously circulate directly in the blood without going through the liver, the body's centre for detoxification.
These findings indicate that intravenous NMN administration is not only safe but also potentially beneficial for humans.
Year:
2022
Link:
Study 4
Study type:
Uncontrolled trial
Purpose:
To investigate the safety of single NMN administration in 10 healthy men
NMN dose:
100, 250, and 500 mg (single administrations)
Duration:
Acute (5 hours)
Results:
The study found an association between NMN administration and a significant increase in the levels of NMN metabolites, the chemical compounds produced as a result of the metabolic processes of NMN. Previous research has already demonstrated a positive correlation between changes in NMN metabolite levels and changes in NAD+, a crucial component in cell functions such as energy production and DNA repair. Therefore, an increase in these NMN metabolites may indicate an elevation in NAD+ levels, potentially benefiting overall health and addressing age-related concerns.
In addition, a single oral administration of NMN up to 500 mg was safe and well-tolerated in healthy men. No significant adverse effects were observed. The authors concluded that oral administration of NMN is feasible and could be used as a potential therapeutic strategy to replenish cellular NAD+ levels to mitigate age-related functional disorders in humans.
Year:
2020
Link:
PRODUCTS CONTAINING NMN
NMN, Resveratrol & Collagen Bundle

Saw Palmetto Scientific Studies
We have summarised the most interesting studies of saw palmetto related to hair loss.
Study 1 (Hair loss)
Study type:
Systematic review
Purpose:
To describe the effects of Saw Palmetto extract on hair loss conditions and its associated side effects.
Intervention under study:
Oral and topical supplements containing 100-320 mg/day Saw Palmetto
Studies Reviewed:
5 randomised controlled trials, 3 prospective cohort studies, and 1 case report
Results:
The systematic review found associations between saw palmetto supplements and positive effects on patients with androgenetic alopecia (male pattern hair loss) and telogen effluvium (temporary hair loss that usually happens after stress, a shock, or a traumatic event), such as improvements in hair quality by 60%, hair density, and hair count by 3.4 to 27%.
Year:
2020
Link:
Study 2 (Hair loss)
Study type:
Clinical trial (Uncontrolled)
Purpose:
To compare the effects of Serenoa repens (saw palmetto) with finasteride on treating male androgenetic alopecia
Dose
320 mg/day of saw palmetto or 1 mg/day finasteride per day
Participants:
100 men aged 20 to 40 years
Duration:
2 years
Results:
The study found an association between that 320 mg/day of saw palmetto and an increase in crown hair growth in 38% of patients. 68% of those treated with finasteride noted an improvement.
Year:
2012
Link:
Green Tea Scientific Studies
We have summarised the most interesting studies on green tea.
Study 1 (Oxidative stress & Diabetes)
Study type:
Randomised, single-blind, controlled trial
Purpose:
To examine the effects of green tea extracts and beverages on body weight, fasting glucose, and lipids, and oxidative stress in obese subjects with metabolic syndrome (which is a medical term for a combination of diabetes, high blood pressure and obesity).
Dose:
870 mg/day of green tea capsules (2 x 435 mg capsules) or 928 mg/day of green tea beverages (4 cups x 232 mg tea bags)
Participants:
35 obese males and females with metabolic syndrome and an average age of 43
Duration:
8 weeks
Results:
The researchers observed that the average body weight and body mass index (BMI) of obese participants in the green tea beverage and supplementation group significantly decreased after 8 weeks. A significant reduction in the biomarkers of oxidative stress (malondialdehyde and 4-hydroxynonenal) was also observed in green tea drinkers but not in the green tea supplementation group. A decrease in oxidative stress is associated with reduced risks of cardiovascular disease.
The study also found that the green tea drinkers were associated with a decreasing trend in LDL-cholesterol and an increasing trend in HDL-cholesterol compared to the control groups. High levels of LDL-cholesterol, also known as “bad” cholesterol, are associated with a higher risk for heart disease and stroke. In contrast, higher levels of HDL-cholesterol is associated with a lower risk of heart disease and stroke.
Year:
2010
Link:
Study 2 (Lifespan)
Study type:
Prospective cohort study
Purpose:
To investigate the associations between green tea consumption and mortality from all causes and specific causes.
Method of evaluation:
The amount of green tea consumption was determined using a food frequency questionnaire. The causes of death were investigated by reviewing the death certificates filed at Ohsaki Public Health Center.
Participants:
40,530 participants from the Ohsaki National Health Insurance Cohort Study
Duration:
11 years of follow-up for all-cause mortality and up to 7 years of follow-up for cause-specific mortality
Results:
The study found an association between increased green tea consumption and lower deaths from all causes and cardiovascular disease, except cancer. The association with lower cardiovascular disease deaths was stronger than that with deaths from all causes. The association with deaths from all causes was stronger in women.
Year:
2006
Link:
Study 3 (Lifespan)
Study type:
Population-based, prospective cohort study
Purpose:
To investigate the association between green tea consumption and death from all causes, cancer, and cardiovascular disease among elderly people.
Method of evaluation:
The frequency of green tea consumption was determined using questionnaires. The causes of death of the deceased subjects were identified using the National Vital Statistics Database from the Ministry of Health, Labour and Welfare of Japan.
Participants:
12,251 men and women aged 65–84 years
Duration:
6 years follow up from December 1999 to March 2006
Results:
The study found that those who consumed seven or more cups of green tea per day were associated with a 55% lower risk of death from all causes and a 75% lower risk of death from cardiovascular disease when compared with those who consumed less than one cup per day. A moderate dose of green tea consumption was also associated with a lower risk of colorectal cancer mortality.
Year:
2009
Link:
Ashwagandha Scientific Studies
Numerous clinical trials have investigated the effects of ashwagandha in humans. We have summarised some of the most important studies related to anxiety, stress, sleep, mood, testosterone, sexual function, muscle strength, energy, immune system, oxidative stress, joint health, hypothyroidism, and tuberculosis.
Study 1
Study type:
Pragmatic randomised controlled trial
Purpose:
To compare the effects of ashwagandha treatment with a standardised psychotherapy on employees with moderate to severe anxiety.
Method of evaluation:
Anxiety levels were assessed using a self-reported questionnaire which measured subjective symptoms of anxiety. Quality of life was also assessed using questionnaires which measured health-related quality of life, physical and mental fatigue, and qualitative patient experiences.
Dose:
600 mg/day of ashwagandha (2 x 300 mg) or placebo
Additional interventions:
In addition to the ashwagandha treatment, participants in the naturopathic care group received an adult multivitamin, lifestyle and nutritional counselling, and engaged in diaphragmatic deep breathing exercises during their treatment sessions.
Participants in the control group received 12 weeks of psychotherapy sessions targeting anxiety, consisting of patient-directed counselling and cognitive-behavioural therapy, along with placebo pills.
Participants:
75 male and female employees with moderate to severe anxiety
Duration:
12 weeks
Results:
The study found a significant association between naturopathic treatment, which included dietary changes and daily intake of 600 mg of ashwagandha, and a reduction in anxiety symptoms. Additionally, the researchers observed that the naturopathic group experienced better outcomes in terms of fatigue, motivation, concentration, and overall well-being compared to the psychotherapy group. Patient-centred assessments also revealed significant reductions in specific symptoms and improvements in mental health, vitality, social functioning, and general health in the naturopathic group.
Year:
2009
Link:
Study 2
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To investigate the stress-relieving and pharmacological activity of ashwagandha extract in stressed adults.
Method of Evaluation:
Anxiety, depression, and stress were assessed using both clinician-administered and self-reported questionnaires, which measured the severity of anxiety, stress, and depression.
Dose:
240 mg/day of standardised ashwagandha extract containing 35% withanolides or placebo
Participants:
60 males and females aged 18 to 65 years
Duration:
60 days
Results:
The study found an association between ashwagandha supplementation and a statistically significant reduction in anxiety symptoms, as measured by Hamilton Anxiety Scale when compared to the placebo. Additionally, a notable but not statistically significant decrease in symptoms of depression, anxiety, and stress, as measured by the Depression, Anxiety, and Stress Scale-21, was observed. Moreover, ashwagandha supplementation was linked to greater reductions in morning cortisol levels, a hormone associated with stress, as well as dehydroepiandrosterone sulphate (DHEAS) levels, a hormone involved in hormone production, compared to the placebo. Reductions of DHEA along with morning cortisol levels may be a marker of decreased stress. The researchers also observed an increase in testosterone levels increased in males but not in females over time, although this change was not statistically significant compared with the placebo.
Year:
2019
Link:
Study 3
Study type:
Randomised, placebo-controlled, between-group trial
Purpose:
To assess the effects of ashwagandha on improving cognitive performance, mood, anxiety, food cravings, and cortisol levels in healthy adults with high perceived stress.
Method of Evaluation:
Anxiety, depression, and stress were assessed using self-reported questionnaires which measured trait anxiety levels, magnitude of depression, anxiety, and perceived stress.
Cognitive ability was assessed using a neurocognitive test which measured verbal memory, visual memory, finger tapping, digit coding, stroop test, attention, and performance.
Dose:
225 mg/day of ashwagandha root and leaf extract or 400 mg/day of ashwagandha root and leaf extract or placebo
Participants:
60 healthy men and women with an average age of 34 years
Duration:
30 days
Results:
The researchers observed significant improvements over time in self-report assessments for anxiety, stress, depression, perceived stress, and food cravings. However, these improvements were not specific to the intervention group, as the main effect for the group and interactions were not significant. Additionally, significant improvements were observed in cognitive flexibility, visual memory, reaction time, psychomotor speed, and executive functioning, with the Ashwagandha groups often out-performing the placebo group. Cortisol levels in the ashwagandha groups also exhibited reductions, with larger effects observed in the 225 mg/day ashwagandha group. In contrast, the placebo group showed a nonsignificant increase in cortisol levels. Increased cortisol levels in short term can enhance the body's ability to respond to stressors by providing a burst of energy, heightened focus, and increased alertness.
Year:
2022
Link:
Study 4
Study type:
Randomised, double-blind, placebo-controlled clinical trial
Purpose:
To evaluate the effects of an aqueous ashwagandha root extract in reducing stress and anxiety in adults.
Method of Evaluation:
Anxiety was assessed using a clinician-based psychological questionnaire which measures intensity of anxiety. Stress and quality of sleep was assessed using self-reported questionnaires which measure perceived stress, and overall sleep quality.
Dose:
250 mg/day of ashwagandha root extract (2 x 125 mg capsules) or 600 mg/day of ashwagandha root extract (2 x 300 mg capsules) or placebo
Participants:
60 male and female participants
Duration:
8 weeks
Results:
The researchers observed that the group taking 600 mg of ashwagandha showed significant reductions in stress and anxiety levels, as well as significant improvement in sleep quality compared to the placebo group. Relative to the value at baseline, there was a statistically significant reduction in the mean serum cortisol level in both the 250mg and 600mg ashwagandha groups compared to the placebo group. Lower levels of cortisol indicate a decrease in stress response within the body. Researchers observed a statistically significant reduction in the stress levels in both treatment groups.
Year:
2019
Link:
Study 5
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To evaluate the effects of ashwagandha root extract on patients with generalised anxiety disorder.
Method of evaluation:
Anxiety was assessed using a clinician-based questionnaire which measured the severity of generalised anxiety disorder.
Dose:
1 g/day of ashwagandha or placebo
Participants:
40 patients with a confirmed diagnosis of generalised anxiety disorder
Duration:
Six weeks
Results:
The study found an association between a daily dose of 1g ashwagandha oral intake and greater reduction in anxiety rating scores. The researchers observed a 14-unit reduction in anxiety scores in the ashwagandha group. In contrast, an 8-unit reduction was observed in the placebo group after six weeks of treatment.
Year:
2020
Link:
Study 6
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To evaluate the effects of ashwagandha extract in patients with anxiety disorders.
Method of Evaluation:
Anxiety was assessed using the Hamilton Anxiety Scale, a clinician-based questionnaire used to measure the severity of anxiety.
Dose:
500 mg/day of ashwagandha (2 x 250 mg tablets) or placebo
Participants:
39 men and women with an average age of 41
Duration:
6 weeks
Results:
The study found a trend for superior anti-anxiety effects of Ashwagandha over the placebo at week 2, and statistically significant superior anti-anxiety effects at week 6.
Ashwagandha was well tolerated and adverse effects were comparable to those observed after the placebo.
Year:
2000
Link:
Study 7
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To assess the effects of a standardised extract of ashwagandha in patients with bipolar disorder.
Method of Evaluation:
Cognitive function was assessed using various cognitive assessments that measure executive functions, processing speed, attention/working memory, memory, psychomotor speed, and social cognition.
Dose:
250 mg/day of ashwagandha extract on the first week and 500 mg/day (2 x 250 mg capsules) for the next 7 weeks or placebo
Participants:
60 males and females diagnosed with bipolar disorder
Duration:
8 weeks
Results:
The study revealed a significant association between ashwagandha extract and notable improvements in specific cognitive tasks after an 8-week treatment period. These tasks included remembering numbers in reverse order, responding quickly to neutral stimuli, and accurately identifying emotions.
Reported adverse events were mild and temporary (5 participants reported diarrhoea and 3 participants reported sleepiness). No participants withdrew from the study or experienced serious adverse events.
Year:
2013
Link:
Study 8
Study type:
Randomised, double-blind placebo-controlled trial
Purpose:
To investigate the effects of ashwagandha root extract in patients with obsessive-compulsive disorder.
Method of Evaluation:
Obsessive-compulsive disorder symptoms were assessed using the Yale-Brown Obsessive Compulsive Scale, a symptom checklist which measures the frequency and severity of the symptoms in OCD patients.
Dose:
120 mg/day of ashwagandha (4 x 30 mg of ashwagandha extract) or placebo
Participants:
30 patients diagnosed with obsessive-compulsive disorder and undergoing treatment with selective serotonin reuptake inhibitors (SSRI) drugs at an adequate dose and duration.
Duration:
6 weeks
Results:
The study found an association between daily oral supplementation of 120 mg of ashwagandha and significantly greater reduction in obsessive-compulsive disorder symptoms. The researchers observed an 8-unit reduction in obsessive-compulsive disorder symptoms in the treatment group, while the placebo group only had a 2-unit reduction.
Year:
2016
Link:
https://doi.org/10.1016/j.ctim.2016.03.018
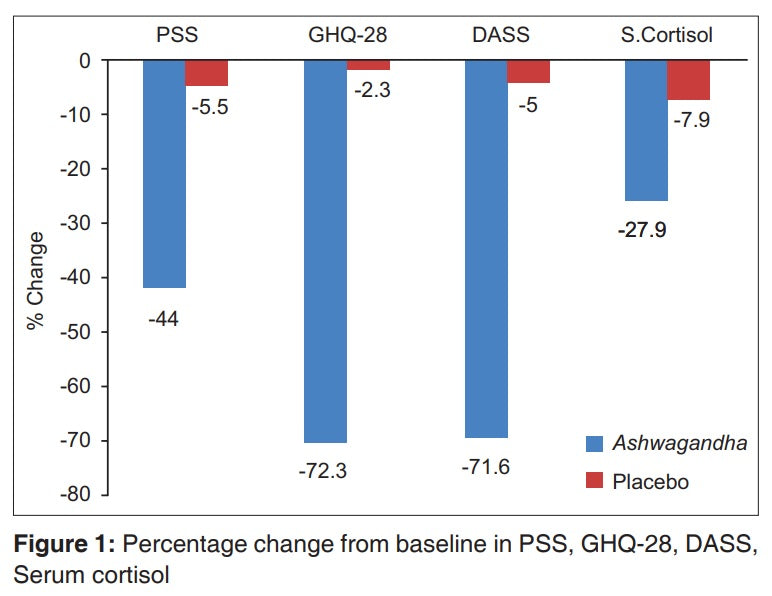
Study 1
Study type:
Single-centred, randomised, double-blind, placebo-controlled study
Purpose:
To evaluate the effects of a high-concentration full-spectrum extract of ashwagandha roots in reducing stress and anxiety and improving the general well-being of stressed adults.
Method of evaluation:
Stress was assessed using self-reported questionnaires which measured various dimensions of stress.
Dose:
600 mg/day of ashwagandha (300 mg x 2 capsules) or placebo
Participants:
64 men and women with history of chronic stress
Duration:
60 days
Results:
The study found an association between the daily dose of 600 mg ashwagandha root extract and a significant reduction in scores on all stress-assessment scales on day 60 compared to the placebo group. Additionally, the researchers observed a reduction in serum cortisol levels in the ashwagandha group which is considered beneficial and indicative of reduced stress levels. No serious adverse events were reported in both groups.
Year:
2012
Link:
Study 2
Study type:
Two-arm, randomised, double-blind, parallel group, placebo-controlled trial
Purpose:
To evaluate the effects of ashwagandha root extract on cognitive functions, stress level, sleep, and quality of life in adults with stress.
Method of Evaluation:
Cognitive function was assessed using the Cambridge Neuropsychological Test Automated Battery which included tests of working memory, learning and executive function, visual, verbal, and episodic memory, attention, information processing, reaction time, social and emotion recognition, decision making, and response control.
Stress, happiness, and sleep quality were assessed using self-reported questionnaires which measured perceived stress, psychological well-being, and quality and pattern of sleep, respectively.
Dose:
300 mg/day of ashwagandha root extract capsules with 15 mg withanolides or placebo
Participants:
125 males and females with an average age of 34 years
Duration:
90 days
Results:
The researchers observed significant improvements in recall memory and a decrease in the total error rate in the ashwagandha treatment group compared to the placebo group. Additionally, the study found associations between ashwagandha treatment and lower stress levels, reduced serum cortisol levels, better psychological well-being, and improved sleep quality. Lower serum cortisol levels suggest reduced stress and improved regulation of the body's stress response.
Year:
2021
Link:
Study 3
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To evaluate the effects of standardised ashwagandha root extract on overall well-being and stress markers linked to obesity in adults with chronic stress.
Method of Evaluation:
Weight management was assessed using body weight, body mass index, and self-reported questionnaires that evaluated stable dimensions of food cravings and eating behaviour. Stress and general well-being were also assessed using self-reported questionnaires that evaluated perceived stress, happiness, well-being, and optimism.
Dose:
600 mg/day of standardised ashwagandha root extract (2 x 300 mg capsules with 5% withanolides) or placebo.
Participants:
52 men and women aged 16 to 60 years, experiencing chronic stress
Duration:
8 weeks
Results:
The study found an association between oral ingestion of 600 mg of ashwagandha root extract and significant decrease in perceived stress levels after 4 and 8 weeks of daily intake. Participants who received the ashwagandha treatment reported feeling happier, experiencing an overall improvement of 19.18%. Additionally, they exhibited improvements in controlling their eating habits and managing emotional eating.
Furthermore, the researchers observed reductions in body weight, body mass index, and serum cortisol levels among those who received the ashwagandha treatment. Serum cortisol levels are indicators of stress and can influence appetite. Lower cortisol levels have the potential to improve appetite control and weight management.
Year:
2015
Link:
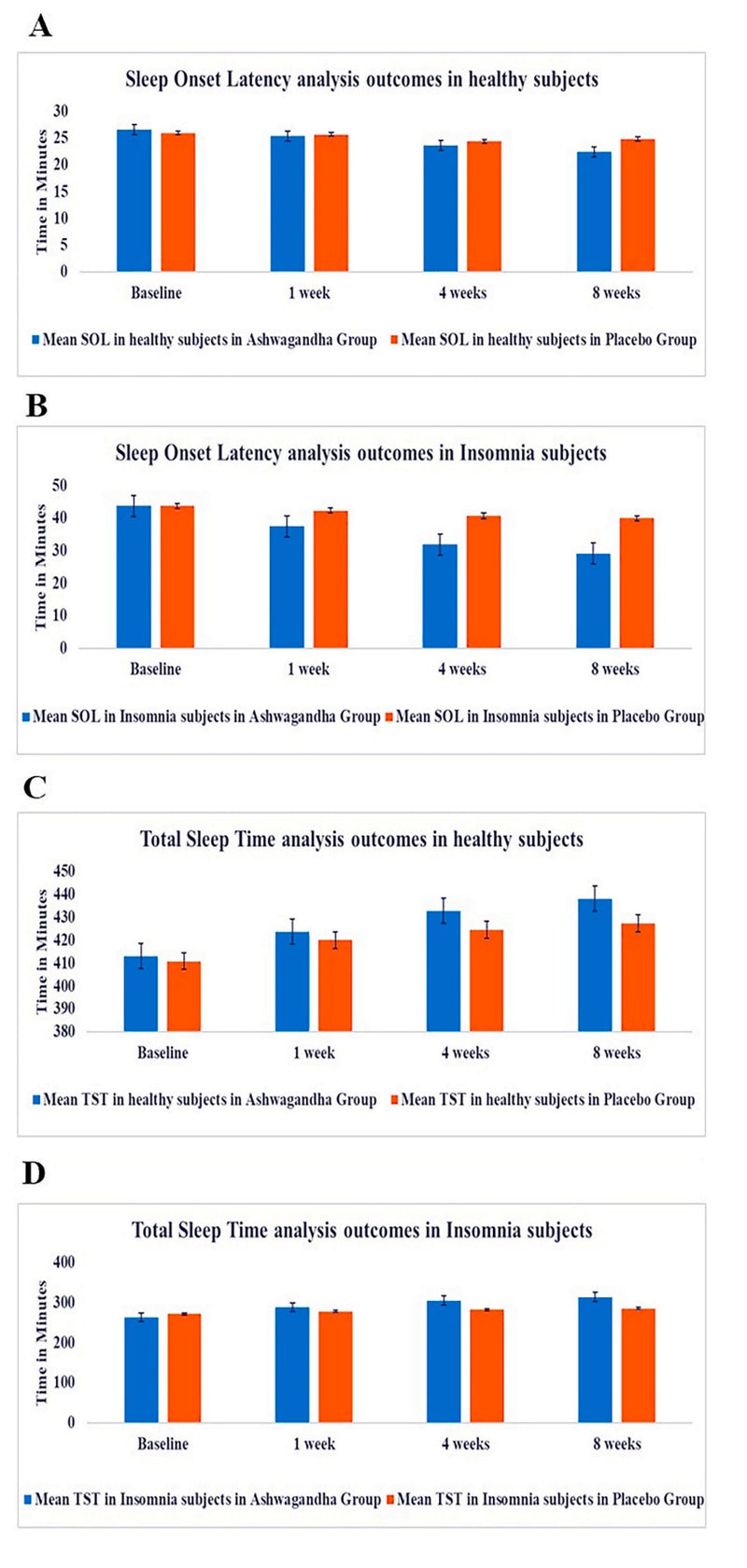
Study 1
Study type:
Randomised, double-blind parallel-group, placebo-controlled study
Purpose:
To compare the effects of ashwagandha root extract between healthy subjects and in subjects with insomnia.
Method of Evaluation:
Sleep parameters were assessed using actigraphy, a non-invasive method of monitoring human rest and activity cycles such as sleep onset latency, total sleep time, wake after sleep onset, total time in bed, and sleep efficiency. In addition, sleep quality was assessed using a self-reported questionnaire, which measured subjective sleep quality.
Anxiety was self-assessed using a psychological questionnaire which measured the severity of perceived anxiety.
Dose:
600 mg/day of ashwagandha root extract (2 x 300 mg capsules) or placebo
Participants:
80 participants with an average age of 37 years
Duration:
8 weeks
Results:
The researchers observed significant improvements in all sleep parameters, including sleep onset latency (the time it takes to fall asleep), total sleep time, wake after sleep onset (the duration of wakefulness during the sleep period), time in bed, and sleep efficiency (the percentage of time spent asleep while in bed). The improvements were more notable in individuals with insomnia compared to healthy subjects, suggesting that ashwagandha treatment may have a greater impact on improving sleep in those with existing sleep difficulties. Moreover, improvements in subjective sleep quality were observed for both the ashwagandha group in the healthy study population and the insomnia study group.
Additionally, the researchers also noticed a notable reduction in anxiety symptoms among individuals with insomnia who received the ashwagandha supplement.
Year:
2021
Link:
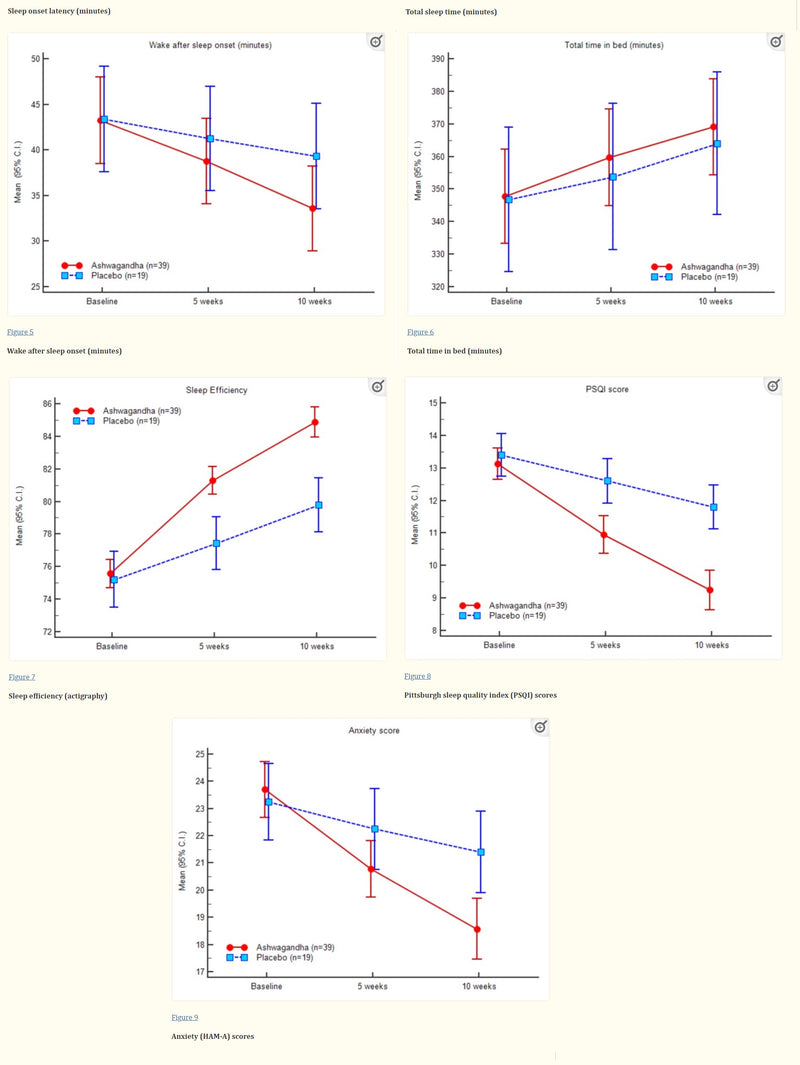
Study 2
Study type:
Randomised, double-blind, placebo-controlled, crossover trial
Purpose:
To determine the effects of ashwagandha root extract in patients with insomnia and anxiety.
Method of Evaluation:
Sleep onset latency and other sleep parameters were assessed by actigraphy, a non-invasive technique used to get an objective measurement of your sleep schedule. Sleep quality was assessed using a self-reported questionnaire and sleep logs which evaluated mental alertness on rising and quality of sleep.
Anxiety was assessed using a self-reported questionnaire which measured mental agitation, psychological distress, and physical complaints related to anxiety.
Dose:
600 mg/day of ashwagandha root extract (2 x 300 mg capsules) or placebo
Participants:
60 males and females aged 18-60 years.
Duration:
10 weeks
Results:
The study found an association between ashwagandha treatment and the significantly shorter sleep onset latency after 10 weeks. The researchers also observed significant improvements in other sleep parameters including total sleep time, sleep efficiency and sleep quality. Furthermore, ashwagandha treatment was associated with lower anxiety levels after 10 weeks of ashwagandha treatment.
Year:
2019
Link:
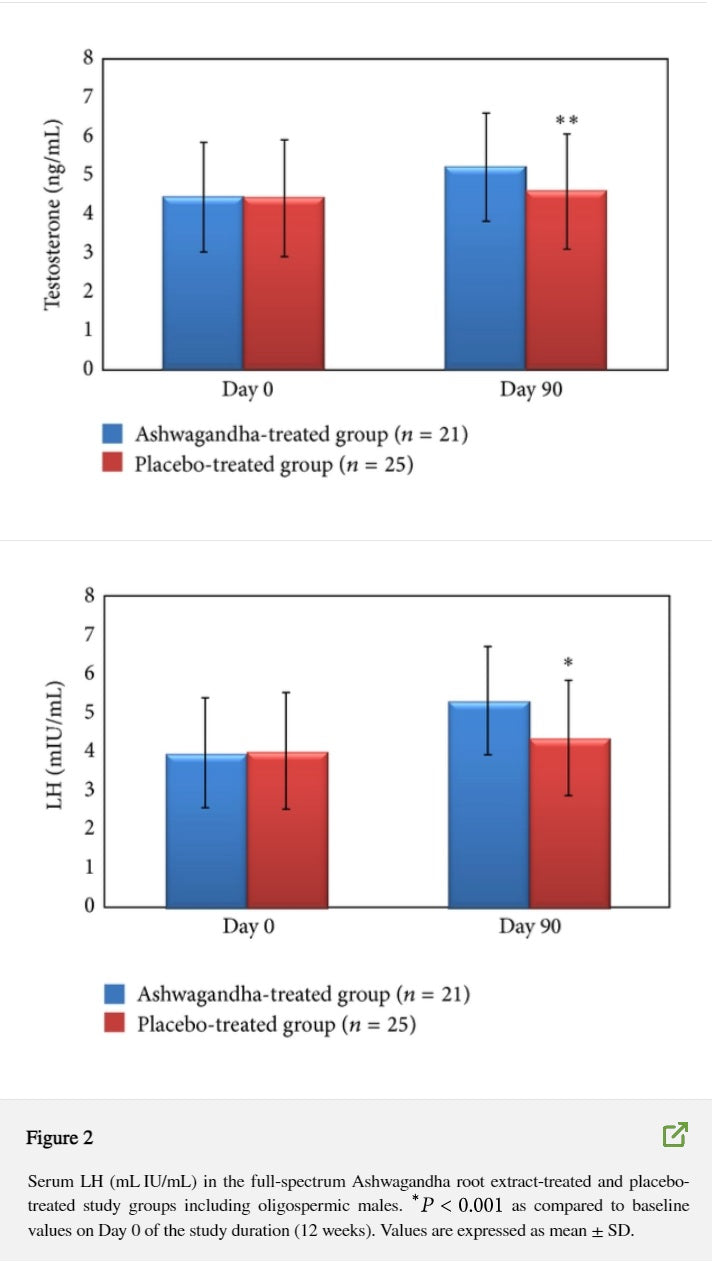
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To assess the effects of ashwagandha root extract on sperm production in patients with oligospermia, or low sperm count.
Dose:
675 mg/day of ashwagandha root extract (3 x 225 mg capsules) or placebo
Participants:
46 infertile male patients aged 22-40 years
Duration:
90 days
Results:
The study found an association between ashwagandha treatment and significant increases in sperm concentration, semen volume, and sperm motility. Additionally, the researchers observed a 17% increase in serum testosterone and a 34% increase in luteinizing hormone following treatment with ashwagandha root extract compared to the baseline. In males, an increase in luteinizing hormone stimulates the production of testosterone in the testes.
Year:
2013
Link:
Study 2
Study type:
Randomised, double-blind placebo-controlled trial
Purpose:
To investigate the effects of ashwagandha on seminal plasma metabolites in infertile men.
Dose:
5 g/day of ashwagandha root powder or placebo
Participants:
180 infertile men in the treatment group and 50 normal healthy fertile men in the control group. Within the treatment group, there were three subgroups of infertile males: 60 men with normal semen parameters but an unknown cause of infertility (normozoospermic), 60 men with low sperm concentration (oligozoospermic), and 60 men with poor sperm motility (asthenozoospermic).
Duration:
3 months
Results:
The study found that ashwagandha treatment was associated with significant increases in the concentrations of alanine, glutamate, citrate, glycerophosphocholine, and histidine, and a decrease in phenylalanine in the normozoospermic group. Similar findings were observed in the oligozoospermic and asthenozoospermic groups after three months of ashwagandha treatment. These improvements in alanine, glutamate, citrate, GPC, and histidine indicate positive effects on male fertility, as these substances are important biomarkers. Additionally, the decrease in phenylalanine suggests potential improvements in overall health and metabolic profile among men.
Year:
2013
Link:
Study 3
Study type:
Randomised, double-blind, placebo-controlled, crossover study
Purpose:
To investigate the effects of ashwagandha supplementation in overweight men with mild-to-moderate, self-reported fatigue.
Method of Evaluation:
Symptomatic changes and mood states were assessed using self-reported questionnaires which measured psychological, somatic, and sexual symptoms, and fatigue inertia and vigour-activity.
Dose:
600 mg/day of ashwagandha extract (2 x 300 mg capsules with 10.5 mg of of withanolide glycosides) or placebo
Participants:
57 overweight males with mild-to-moderate symptoms of fatigue aged 40-70 years
Duration:
16 weeks
Results:
The researchers observed significant improvements in most symptom scores from baseline to week 8 for both the placebo and ashwagandha conditions. In the placebo group, improvements were observed in psychological, somatic, and sexual symptoms, as well as fatigue-inertia and vigour-activity. Similar improvements were found in the ashwagandha group, except for fatigue-inertia scores which did not show significant improvement. The study also found that daily supplementation of 600 mg ashwagandha was associated with an 18% higher increase in salivary dehydroepiandrosterone sulphate (DHEA-S) and a 14.7% higher increase in testosterone compared to the placebo group. Higher concentrations of DHEA-S are associated with improvements in mood and reductions in fatigue.
Year:
2019
Link:
Study 4
Study type:
Triple-blind, randomised clinical trial
Purpose:
To compare the effects of ashwagandha and pentoxifylline on sperm parameters in idiopathic male infertility.
Dose:
5 g/day of ashwagandha extract (6 x 0.83 g capsules) or 800 mg/day of pentoxifylline (6 x 133.33 mg capsules) and placebo
Participants:
100 infertile male patients with an average age of 34 years old
Duration:
90 days
Results:
The researchers observed a significant increase of 12.5% in average sperm count, 21.42% in progressive motility, and 25.56% improvement in sperm morphology with ashwagandha supplementation compared to baseline measurements. Similarly, a significant increase of 16.46% in average semen volume, 25.97% in progressive motility, and 13.28% improvement in sperm morphology compared to baseline was observed with pentoxifylline. However, no statistical differences were found between the treatment groups.
Year:
2018
Link:
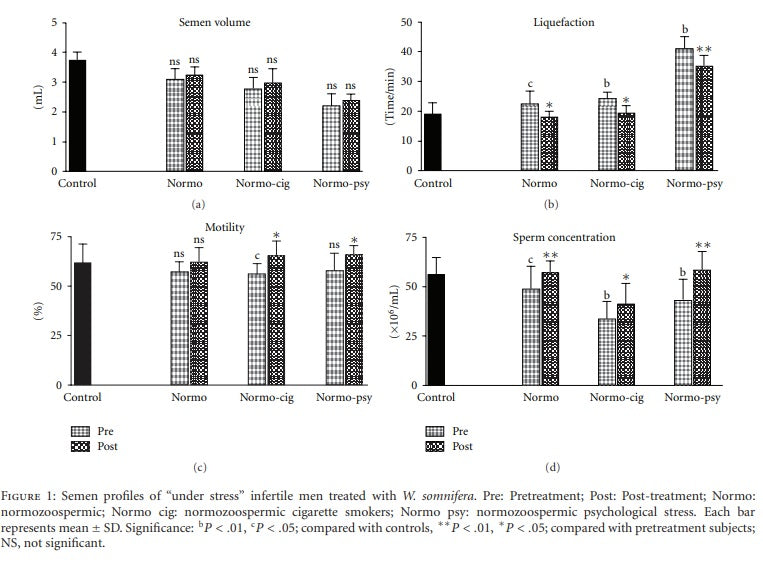
Study 5
Study type:
Randomised clinical trial (uncontrolled)
Purpose:
To explore the potential protective effects of ashwagandha in infertile men who were either experiencing psychological stress or were smokers.
Dose:
5 g/day of ashwagandha root powder with a cup of skimmed milk
Participants:
121 men, aged 25-38 years
Duration:
3 months
Results:
The study found an association between ashwagandha treatment and improved semen parameters in men. The researchers observed a 17% increase of sperm concentration in men with normal sperm parameters (normozoospermic), 20% in cigarette smokers, and 36% in men experiencing psychological stress. The movement of sperm also improved by 9%, 10%, and 13% in the respective groups along with decrease in their semen liquefaction time by 19, 20 and 34%, as compared with the pretreatment parameters. A decrease in semen liquefaction time improves the chances of successful fertilisation.
In addition, the researchers examined the pregnancy outcomes of the men's partners and observed a 15% success rate for men with normal sperm parameters, 15% for men experiencing psychological stress, and 10% for cigarette smokers, leading to an overall success rate of 14%.
Year:
2009
Link:
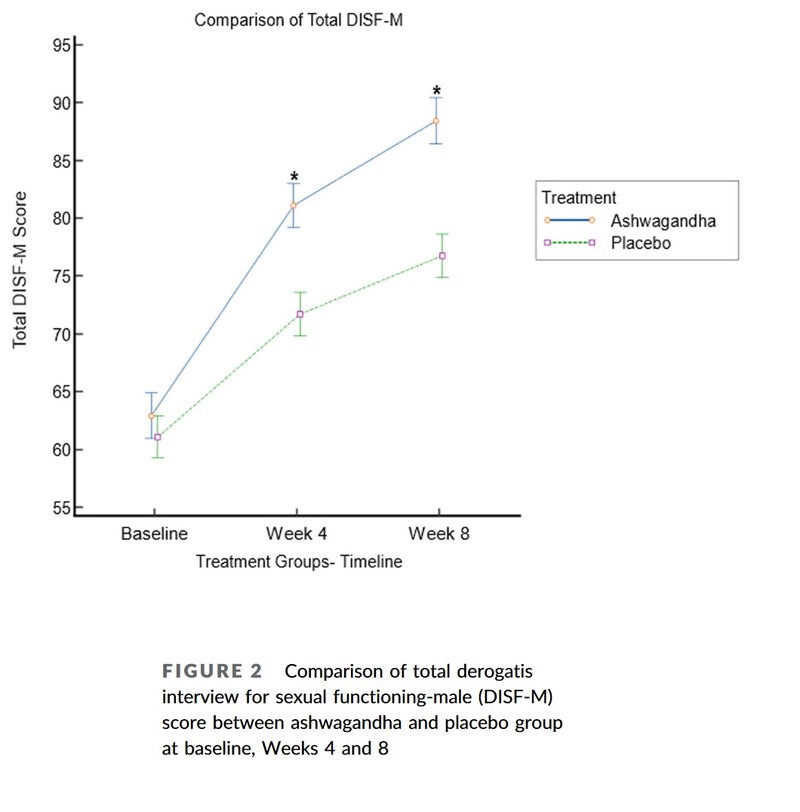
Study 1
Study type:
Randomised, double-blind, placebo-controlled study
Purpose:
To evaluate the effects of ashwagandha root extract on sexual performance and well-being in adult males.
Method of Evaluation:
Sexual activity and quality of sexual function were assessed using a self-reported questionnaire which included questions related to sexual cognition/fantasy, sexual arousal, sexual behaviour/experiences, orgasm, and drive/desire. Quality of life was also assessed using a self-reported questionnaire that evaluated physical functioning, emotional well-being, pain, energy, and fatigue.
Dose:
600 mg/day of ashwagandha root extract (2 x 300 mg capsules) or placebo
Participants:
50 healthy males aged 21-45 years
Duration:
8 weeks
Results:
Participants who took the ashwagandha root extract had an 88.5% greater probability of scoring higher on questionnaires about their sexual activity and quality of sexual function. Additionally, the researchers observed a 17% rise in serum testosterone in the ashwagandha group, while the placebo group only had a 2% change. Participants in the ashwagandha group also showed slight improvement and stability in quality of life parameters compared to the placebo group, although there were no statistically significant improvements observed for either group.
Year:
2022
Link:
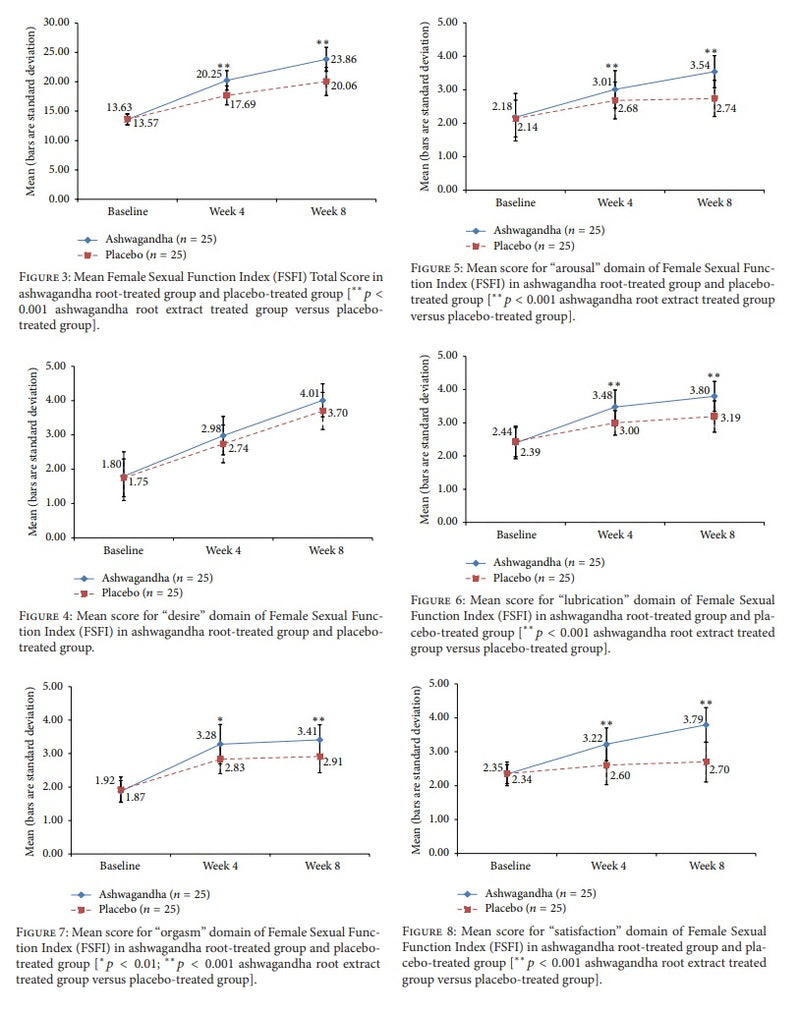
Study 2
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To investigate the effects of ashwagandha root extract supplementation on improving sexual function in healthy females.
Method of Evaluation:
Female sexual function was assessed using self-reported questionnaires which measured sexual desire, arousal, lubrication, orgasm, satisfaction, pain, distress, and sexual activity.
Dose:
600 mg/day of high-concentration ashwagandha root extract (2 x 300 mg capsules) or placebo
Participants:
50 women aged 21-50 years
Duration:
8 weeks
Results:
The study found an association between high-concentration ashwagandha root extract supplementation and a significant increase in female sexual function. Specifically, improvements were observed in lubrication, orgasm, and sexual distress. The average number of successful sexual encounters also showed a significant increase, with a 96% improvement at week 4 and a 126% improvement at week 8, compared to the 59-61% increase observed in the placebo group.
Year:
2015
Link:
Study 1
Study type:
Randomised, double-blind placebo-controlled study
Purpose:
To evaluate the effects of ashwagandha root extract on climacteric symptoms, quality of life, and hormonal parameters in perimenopausal women.
Method of Evaluation:
Menopausal symptoms were assessed using self-reported questionnaires, which measured the severity of menopausal symptoms and their impact on various aspects of a woman's life, including vasomotor symptoms (hot flashes), psychosocial, physical, and sexual symptoms.
Dose:
600 mg/day of ashwagandha root extract (2 x 300 mg capsules) or placebo
Participants:
100 women with climacteric symptoms
Duration:
8 weeks
Results:
The study found an association between 600 mg of ashwagandha daily intake and improvement in menopause-specific quality of life. Additionally, the researchers observed a significant increase in serum estradiol. An increase in serum estradiol levels can have several implications, such as supporting reproductive health, promoting bone density, and influencing mood and cognitive function.
Year:
2021
Link:
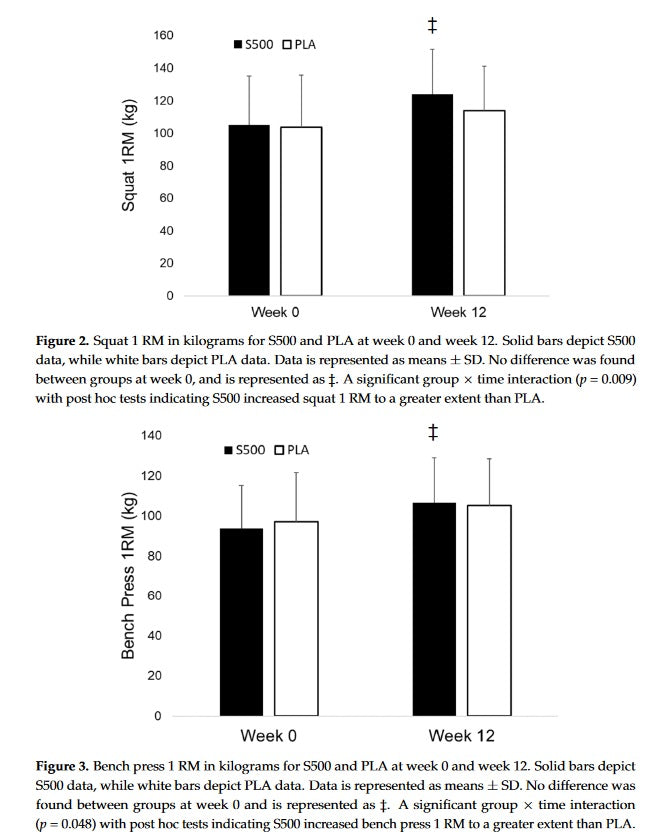
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To investigate the effects of a standardised ashwagandha (Sensoril) root and leaf extract on strength training adaptations and recovery.
Method of Evaluation:
Muscular strength was measured through one-repetition bench press and back squat using standard methods
Dose:
500 mg/day of ashwagandha supplementation or placebo taken every morning with 12 fluid ounces of cold tap water
Additional interventions:
4 days/week resistance-training program designed to train the upper body and lower body
Participants:
40 males with an average age of 26 years
Duration:
12 weeks
Results:
The study found that the daily dose of 500 mg ashwagandha extract, combined with a heavy resistance-training program is associated with the significant improvements in lower-body and upper-body strength after 12 weeks. Additionally, the researchers observed significant improvements in squat power, bench press power, 7.5 km time trial performance, and perceived recovery scores in the ashwagandha group but not in placebo.
Furthermore, no change in the android/gynoid ratio was observed in the ashwagandha group, whereas the placebo group experienced a significant increase in android/gynoid ratios. Higher android/gynoid ratios are associated with an increased risk of health conditions such as cardiovascular disease and metabolic disorders.
Year:
2018
Link:
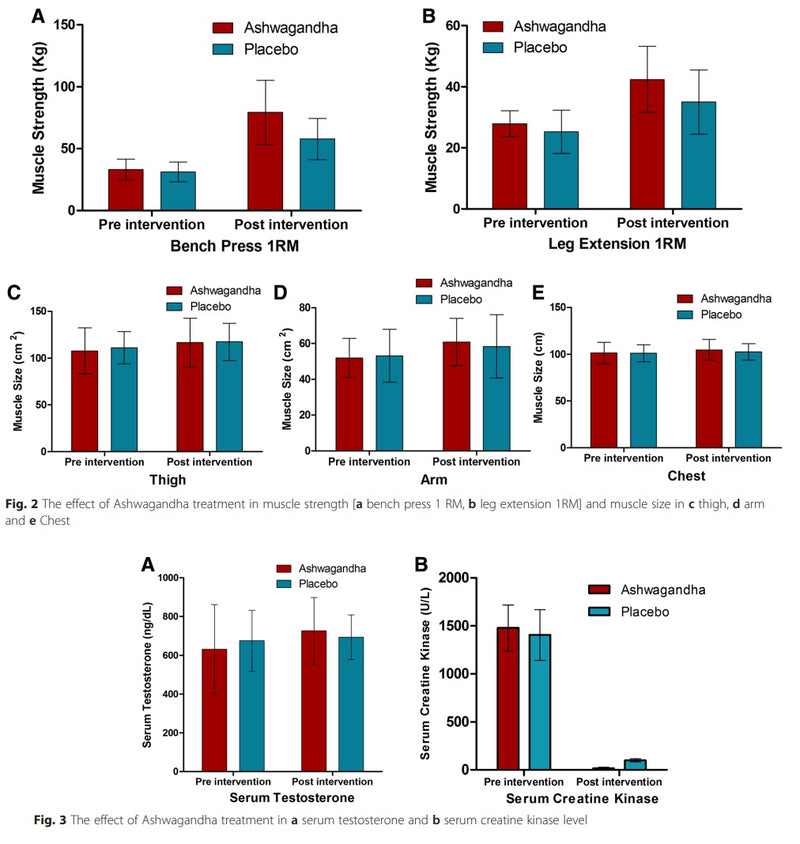
Study 2
Study type:
Randomised, Prospective, Double-Blind, Placebo-Controlled Clinical Trial
Purpose:
To examine the effects of ashwagandha root extract on muscle mass and strength in healthy young men engaged in resistance training.
Method of evaluation:
Muscular strength was measured through one-repetition leg and bench press using standard methods.
Dose:
600 mg/day of ashwagandha (2 x 300 mg capsules with 5% withanolides) or placebo
Additional interventions:
Resistance training program in both upper body and lower body
Participants:
57 young males aged 18-50 years with little experience in resistance training
Duration:
8 weeks
Results:
The researchers observed significant improvements in muscle strength and size in both the ashwagandha group and placebo group, which can be expected with resistance training. However, the ashwagandha group showed significantly greater increases in muscle strength for both the upper and lower body compared to the placebo group. Additionally, the ashwagandha group experienced greater improvements in muscle size, muscle recovery, and a decrease in body fat percentage compared to the placebo group. The study also found an association between ashwagandha treatment and significantly greater increase in serum testosterone.
Year:
2015
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To evaluate the effects of ashwagandha root extract on cardiorespiratory endurance in healthy athletic adults.
Method of Evaluation:
Cardiorespiratory fitness was assessed using Cooper’s 12-minute run test which measured maximum oxygen consumption (VO₂ max).
Dose:
600 mg/day of ashwagandha (2 x 300 mg capsules) or placebo
Participants:
50 healthy athletic adults with an average age of 29 years
Duration:
8 weeks
Results:
The study found an association between ashwagandha supplementation and statistically significant improvements in antioxidant levels and VO₂ max compared to the placebo group. Higher values of VO₂ max indicate better aerobic fitness.
In addition, the researchers observed significant improvements in various assessments of recovery, well-being, and performance in the ashwagandha group compared to the placebo group. The TQR (Total Quality Recovery) scores indicated better overall recovery and well-being in the Ashwagandha group. The DALDA (Daily Analysis of Life Demands for Athletes) scores, which measures different aspects of performance, also favoured the ashwagandha group. The RESTQ (Recovery-Stress Questionnaire for Athletes) assessment showed better outcomes in terms of fatigue recovery, lack of energy, and fitness analysis in the ashwagandha group.
Year:
2019
Link:
Study 2
Study type:
Randomised, controlled, parallel group, single-blinded trial
Purpose:
To investigate the effects of ashwagandha and terminalia arjuna, both individually and in combination, on physical and cardiovascular performance in healthy young adults.
Dose:
500 mg/day of ashwagandha and/or 500 mg/day of Terminalia arjuna or placebo
Participants:
40 healthy males and females with an average age of 21 years
Duration:
8 weeks
Results:
The researchers observed significant improvements in velocity, power, and maximal aerobic capacity (VO₂ max) in participants taking ashwagandha, while those who took Terminalia arjuna had increased VO₂ max and lower resting systolic blood pressure. VO₂ max is a measure of the body's oxygen usage during exercise and higher values indicate better fitness. In addition, a lower resting systolic blood pressure is generally considered beneficial for cardiovascular health. When the two supplements were administered together, improvements were seen in all physical performance and endurance parameters except balance and diastolic blood pressure.
Year:
2010
Link:
Study 3
Study type:
Randomised, placebo-controlled trial
Purpose:
To investigate the effects of ashwagandha supplementation in enhancing the aerobic performance of elite Indian cyclists.
Method of Evaluation:
Aerobic capacity in terms of maximal aerobic capacity (VO₂ max), metabolic equivalent, respiratory exchange ratio (RER), and total time for the athlete to reach his exhaustion stage was determined during a treadmill test in which subjects were asked to perform till volitional exhaustion.
Dose:
1,000 mg/day of Ashwagandha (2 x 500 mg capsules) or a placebo
Participants:
40 elite Indian cyclists aged 18-27 years
Duration:
8 weeks
Results:
The study found a significant association between ashwagandha treatment and improvements in all parameters related to aerobic capacity, such as VO₂ max, metabolic equivalents of tasks (METs), and time to exhaustion on the treadmill.These findings indicate that ashwagandha supplementation can enhance endurance and cardiovascular fitness. VO₂ max measures the body's maximum ability to utilise oxygen during intense exercise, and higher values indicate better aerobic fitness. METs, on the other hand, provide a measure of the intensity of physical activity, and improvements in METs suggest increased aerobic performance and overall fitness levels.
Year:
2012
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial with an open-label extension
Purpose:
To evaluate the effects of ashwagandha extract in middle-aged healthy individuals exposed to environmental influences of seasonal change.
Dose:
180 mg/day capsule containing 60 mg of ashwagandha with 21 mg withanolides or placebo
Participants:
24 healthy healthy men and women aged 45-72 years
Duration:
30 days and an open-label extension study for another 30 days
Results:
The study found an association between ashwagandha treatment and significant improvements in the participant’s immune system after 30 days. They had higher levels of certain immune markers, such as antibodies (IgA, IgM, IgG), cytokines (IFN-γ, IL4), and different types of immune cells (TBNK cells). Higher levels of antibodies, cytokines, and immune cells indicate an enhanced immune response. In contrast, the group that received a placebo had a decrease in immune cells and no change in antibody levels or cytokines.
Year:
2021
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To compare the effect of ashwagandha and guduchi on oxidative stress in healthy volunteers
Dose:
1,000 mg/day of ashwagandha (2 x 500 mg capsules) or 1,000 mg/day guduchi (2 x 500 mg capsules) or placebo
Participants:
30 males and females aged 18-45 years
Duration:
6 months
Results:
The study found that ashwagandha treatment was associated with increased haemoglobin levels. Higher levels of haemoglobin can indicate factors such as improved oxygenation and increased red blood cell production. Additionally, the researchers observed a significant increase in the level of superoxide dismutase and a decrease in the level of malondialdehyde. An increase in superoxide dismutase levels, as observed in the study, suggests that the body's antioxidant defence system is enhanced. It indicates a potential improvement in the body's ability to cope with oxidative stress. In contrast, a decrease in malondialdehyde levels reflects a decrease in the harmful effects of oxidative stress.
No adverse effects were found in the trial period of 6 months.
Year:
2014
Link:
Study 1
Study type:
Prospective, randomised, double-blind, placebo-controlled trial
Purpose:
To evaluate the effects of ashwagandha root and leaf extracts in patients with knee joint pain and discomfort.
Method of Evaluation
Knee joint pain and discomfort were assessed using a self-administered questionnaire which measures pain, stiffness, and physical functional disability in patients with hip and knee osteoarthritis.
Dose:
250 mg/day of ashwagandha (2 x 125 mg capsules) or 500 mg/day of ashwagandha (2 x 250 mg capsules) or placebo
Participants:
60 males and females with knee joint pain and discomfort with an average age of 58 years
Duration:
12 weeks
Results:
The researchers observed that both daily doses of 250 mg and 500 mg of ashwagandha taken over a period of 12 weeks resulted in significant improvements in various measures of osteoarthritis, including the osteoarthritis index score (mWOMAC), knee swelling, pain, stiffness, and disability when compared to baseline and placebo. When comparing the two doses, the 500 mg daily dose of ashwagandha showed better outcomes. Additionally, the 500 mg/day dose showed earlier effects at 4 weeks compared to the 250 mg/day dose.
Year:
2016
Link:
Study 1
Study type:
Randomised, Double-Blind, Placebo-Controlled Clinical Trial
Purpose:
To evaluate the effects of ashwagandha root extract in patients with subclinical hypothyroidism.
Dose:
600 mg/day of ashwagandha (2 x 300 mg capsules containing 5% withanolides) or placebo
Participants:
50 males and females with subclinical hypothyroidism, compared to baseline aged 18-50 years
Duration:
8 weeks
Results:
Supplementation with ashwagandha root extract for 4 and 8 weeks was associated with a significant increase in serum T3 levels, indicating higher concentrations of this thyroid hormone in the bloodstream. The placebo group, on the other hand, experienced a decrease in serum T3 levels over time. Similarly, ashwagandha treatment was associated with a significant increase in serum T4 concentrations at both the fourth and eighth weeks. T3 and T4 are important hormones for regulating metabolism and energy production in the body. Furthermore, the ashwagandha group exhibited a significant decrease in serum TSH levels compared to the placebo group. A decrease in TSH suggests improved thyroid function and a more balanced production of thyroid hormones. Overall, these findings indicate that ashwagandha supplementation may have positive effects on thyroid hormone levels in individuals with subclinical hypothyroidism.
Year:
2018
Link:
Study 1
Study type:
Randomised, double-blind, placebo control trial
Purpose:
To examine the impact of ashwagandha when used alongside a short course treatment in individuals newly diagnosed with sputum smear-positive pulmonary tuberculosis.
Dose:
1000 mg/day of ashwagandha root extract (2 x 500 mg capsules) or placebo
Additional Interventions:
First line antitubercular drugs (drugs used to treat tuberculosis)
Participants:
60 newly diagnosed sputum smear-positive patients of pulmonary TB of category 1
Duration:
12 weeks
Results:
After 8 weeks of treatment, the researchers observed sputum conversion (when the patient's sputum tests negative for the bacteria after a period of treatment) in 86.6% of patients taking ashwagandha as an adjuvant in conjunction with anti-TB drugs, compared to 76.6% in the placebo group. Sputum conversion is a positive response to treatment and indicates a reduction in the bacterial load in the respiratory system. The study also found an association between ashwagandha treatment and the significant increase in CD4 and CD8 counts. An increase in CD4 and CD8 counts refers to an increase in the number of specific types of immune cells called T cells in the blood which may indicate that the immune system is successfully fighting the infection and working to control the disease.
In terms of liver function, a smaller percentage of patients in the ashwagandha group had increased levels of SGOT (serum glutamic oxaloacetic transaminase) and SGPT (serum glutamic pyruvic transaminase), which are enzymes that indicate liver health, compared to the placebo group. This is a positive result, as it shows that the treatment may be gentler on the liver. Furthermore, elevated levels of serum uric acid (>6 mg/dl) were observed in 20% of patients in the study group and 33.33% in the placebo group. Elevated uric acid levels can sometimes indicate certain health issues.
Lastly, patients in the ashwagandha group reported a better overall improvement in their quality of life compared to the placebo group.
Year:
2018
Link:
PRODUCTS CONTAINING ASHWAGANDHA
Ashwagandha Withania Somnifera - KSM-66 Ashwagandha

-
 Testosterone BoostTestosterone Boost Rating 5/10.05/10
Testosterone BoostTestosterone Boost Rating 5/10.05/10 -
 Muscle GrowthMuscle Growth Rating 6/10.06/10
Muscle GrowthMuscle Growth Rating 6/10.06/10 -
 Strength GainsStrength Gains Rating 7/10.07/10
Strength GainsStrength Gains Rating 7/10.07/10 -
 Erection ImprovementErection Improvement Rating 2/10.02/10
Erection ImprovementErection Improvement Rating 2/10.02/10 -
 Libido BoostLibido Boost Rating 1/10.01/10
Libido BoostLibido Boost Rating 1/10.01/10 -
 Stress ReductionStress Reduction Rating 10/10.010/10
Stress ReductionStress Reduction Rating 10/10.010/10
Lemon Balm Scientific Studies
We have summarised some of the most interesting scientific studies on lemon balm.
Study 1
Study type:
Randomised, double-blind, placebo controlled trial
Purpose:
To assess the effects of lemon balm on sleep quality of postmenopausal women. Research has shown that sleep disorder is one of the most common problems in menopausal women.
Method of evaluation:
Sleep quality was assessed using a self-reported questionnaire called the Pittsburgh Sleep Quality Index, which measures seven areas: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction.
Dose:
500 mg/day lemon balm extract (2 x 250 mg capsules) or placebo
Participants:
110 postmenopausal women with an average age of 53 years
Duration:
1 month
Results:
The researchers observed a significant improvement in sleep quality in the lemon balm group compared to the control group after 1 month of intervention. These results suggest that lemon balm may be a beneficial, natural option for enhancing sleep quality over a relatively short period of time.
Year:
2020
Link:
Study 2
Study type:
Randomised, double-blind, placebo controlled trial
Purpose:
To determine the effects of lemon balm supplementation on depression, anxiety, stress, and sleep disturbances in patients with chronic stable angina, a type of chest pain that happens during physical activity or stress due to reduced blood flow to the heart.
Method of evaluation:
Sleep quality was assessed before and after the intervention using the Pittsburgh Sleep Quality Index, a self-reported questionnaire which measures seven areas: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction.
Dose:
3,000 mg/day of lemon balm (3 x 1,000 mg capsules) or placebo
Participants:
73 men and women with an average age of 58 years
Duration:
8 weeks
Results:
The researchers observed a significant decrease in the total sleep disorder score in the intervention group compared to the control group after treatment. Specifically, the intervention group demonstrated better sleep quality, longer sleep duration, and improved sleep efficiency compared to the control group.
Year:
2018
Link:
Study 3
Study type:
Randomised, double-blind, placebo controlled trial
Purpose:
To evaluate the effects of lemon balm on anxiety and sleep quality in patients undergoing coronary artery bypass surgery. Studies have shown that anxiety and sleep disorders are common following surgery, often affecting recovery and overall well-being.
Method of evaluation:
Sleep quality was assessed using a self-reported questionnaire called St. Mary’s Hospital Sleep Questionnaire, which measures the severity of sleep disorder.
Dose:
1500 mg/day lemon balm (3 x 500 mg capsules) or placebo
Participants:
80 men and women with an average age of 58 years
Duration:
7 days
Results:
The participants in the lemon balm group showed a significantly greater improvement in sleep quality and lower anxiety scores compared to the placebo group. Overall, the researchers observed that lemon balm improved sleep quality by 54% and reduced anxiety by 49% in patients after coronary artery bypass surgery.
Year:
2019
Link:
Study 4
Study type:
Open-label pilot trial (uncontrolled)
Purpose:
To evaluate the effects of a lemon balm extract on stressed volunteers with mild-to-moderate anxiety disorders and sleep disturbances.
Method of Evaluation:
Anxiety and insomnia was assessed using questionnaires which measure anxiety manifestations, anxiety-associated symptoms and insomnia.
Dose:
600 mg/day of lemon balm extract (2 x 300 mg tablets; 300mg in the morning and 300 mg before bed)
Participants:
20 stressed male and female volunteers aged 18-70 years
Duration:
15 days
Results:
The study found an association between 600 mg of lemon balm and significant reductions in anxiety symptoms, with a 15% decrease in anxiety-related symptoms, an 18% reduction in anxiety manifestations, and a 42% improvement in insomnia. Additionally, there was a decrease in agitation, hyper-excitation, and other anxiety-related issues, including eating problems, guilt, and fatigue.
Year:
2011
Link:
Study 1 (Stress)
Study type:
Randomised, double-blind, placebo-controlled, balanced crossover trial
Purpose:
To investigate the effects of Lemon Balm on participants exposed to laboratory-induced stress
Dose:
300 mg of lemon balm (2 x 150 mg capsules), 600 mg (4 x 150 mg capsules) of lemon balm, and placebo
Participants:
10 males and 18 females with an average age of 29 years
Duration:
300 mg lemon balm or 600 mg lemon balm or a placebo, with a 7-day interval between each dose.
Results:
The study found an association between a single 600 mg dose of lemon balm and a significant increase in self-reported calmness and a significant reduction in self-reported alertness after exposure to stress compared to a placebo. The study also found that a single 300 mg dose of lemon balm is associated with the significant increase in the speed and accuracy of mathematical processing compared to placebo.
Year:
2004
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To examine the effects of Melissa officinalis (Lemon balm) on the psychological health of female adolescents with premenstrual syndrome
Method of Evaluation:
Psychological health was self-assessed using the General Health Questionnaire, which included questions related to depression, anxiety, sleeping and social function disorder, and somatoform symptoms.
Dose:
1200 mg/day of Lemon balm essence (2 x 600 mg capsules) or placebo
Participants:
100 females with premenstrual syndrome (aged 16 on average)
Duration:
3 menstrual period cycles (7 days a month for 3 months)
Results:
The researchers observed significantly lower scores for anxiety, sleep disturbances, social function disturbance, psychosomatic symptoms and depression in participants who took 1,200 mg/day of Lemon balm during their menstrual period for 3 cycles compared to the placebo group.
Year:
2017
Link:
Study 4 (Sexual Function)
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To evaluate the effects of lemon balm extract on women with sexual dysfunction.
Method of Evaluation:
Sexual function was self-reported using the Female Sexual Function Index (FSFI) questionnaire, which evaluated their sexual function in terms of desire, arousal, lubrication, orgasm, satisfaction, and pain.
Dose:
1000 mg/day of Lemon balm extract (2 x 500 mg tablet; 500 mg an hour after breakfast and 500 mg an hour before dinner) or placebo
Participants:
43 women with an average age of 35 years
Duration:
4 weeks
Results:
The study found an association between 1000 mg/day lemon balm extract and significant improvements in all sexual domains (desire, arousal, lubrication, orgasm, satisfaction, and pain) after a month of intake. The average amount of sexual intercourse in a month increased by 9.0 in people receiving 1000 mg/day lemon balm, nearly twice as much as the placebo group (4.3). In addition, 90% in the Lemon balm treatment group were significantly more willing to continue treatment as compared to those in the placebo group.
Year:
2018
Link:
Turmeric Scientific Studies
We have summarised the most interesting studies of turmeric related to arthritis, depression, and chronic pain.
Study 1 (Arthritis)
Study type:
Randomised, double-blind, placebo-controlled, parallel trial
Purpose:
To evaluate the effects of turmeric on the clinical symptoms of rheumatoid arthritis
Dose:
500 mg/day of curcumin (2 x 250 mg capsules), 1000 mg/day of curcumin (2 x 500 mg capsules) or placebo
Participants:
36 males and females with diagnosed rheumatoid arthritis
Duration:
90 days
Results:
The study found that 500 mg and 1000 mg of curcumin were associated with significant improvements in the symptoms of rheumatoid arthritis, with more significant reductions in the high-dose treatment group (1000 mg/day). The researchers observed reductions in swollen joints by 85% and overall tender joints by 88% in the high-dose treatment group compared to the low-dose treatment group (80% and 78%, respectively).
Biomarkers of rheumatoid arthritis (including erythrocyte sedimentation rates, C-reactive protein, and rheumatoid factor) also decreased significantly in both treatment groups.
No adverse effects were observed or reported.
Year:
2017
Link:
Study 2 (Anxiety & mood)
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To investigate the effects of curcumin supplementation on individuals with major depressive disorder
Method of Evaluation:
Depressive symptoms and anxiety were self-reported using questionnaires.
Dose:
1000 mg/day of curcumin (2 x 500 mg capsules) or placebo
Participants:
56 males and females aged 18 to 65 years
Duration:
8 weeks
Results:
The study found that 1000 mg/day of curcumin and placebo were associated with reductions in depressive and anxiety symptoms in the first 4 weeks of treatment. From weeks 4 to 8, curcumin was significantly more effective than placebo in improving several mood-related symptoms.
Year:
2014
Link:
Study 3 (Chronic Pain)
Study type:
Pilot comparative study
Purpose:
To compare the pain-relieving properties of curcumin and two painkilling drugs in subjects affected by acute pain.
Dose:
1.5 g/day of Meriva (3 x 500 mg pills with 20% curcumin) or 2.0 g/day of Meriva (4 x 500 mg pills with 20% curcumin) or 100 mg/day nimesulide or 1 g/day acetaminophen
Participants:
15 males and females with an average age of 50 years
Duration:
8 days (2 cycles) with 24-48 hour discontinuance cycle between treatments
Results:
The study showed that a 2g lecithin formulation containing 400mg of curcumin was associated with a reduction in pain perception 2 hours after administration, and the painkilling (analgesic) effect lasted for 4 hours. This activity was comparable but slightly higher than that of 1 g acetaminophen (paracetamol) but lower than the effect of 100 mg nimesulide.
Year:
2013
Link:
Milk Thistle Scientific Studies
We have summarised some of the most interesting scientific studies on milk thistle and silymarin. Silymarin is the main active component of milk thistle.
Study 1 (Liver Disease)
Study type:
Randomised controlled trial
Purpose:
To determine the effect of silymarin (the main active ingredient in milk thistle extracts) on patients with cirrhosis
Dose:
420 mg/day of silymarin (3 x 140 mg capsules) or placebo
Participants:
170 male and female patients with cirrhosis of the liver
Duration:
2 years
Results:
The researchers observed a higher survival rate in the 420 mg/day silymarin treatment group (82%) compared to placebo (68%). An association was found between silymarin treatment and a 58% survival rate 4 years after the trial, compared to only 38% in the placebo group. Survival differences were most evident in patients with alcohol-related liver disease, cirrhosis, and those with low-severity disease.
Year:
1989
Link:
Study 2 (Liver Disease)
Study type:
Randomised controlled double-blind trial
Purpose:
To investigate the effects of silymarin (the main active ingredient in milk thistle extracts) on the chemical, functional, and morphological features of liver disease.
Dose:
420 mg/day silymarin or placebo
Participants:
97 in-patients of the Central Military Hospital
Duration:
4 weeks
Results:
The study found an association between 420 mg/day of silymarin and significant decreases in serum alanine aminotransferase and serum aspartate amino-transferase (higher levels of these liver enzymes may indicate liver damage).
Sulfobromophthalein (a dye used in liver function tests, whereby the rate of removal of the dye from the bloodstream gives a measure of liver function) retention also returned to normal significantly more often in the silymarin group.
Year:
1982
Link:
Study 3 (Liver Disease)
Study type:
Randomised, double-blind, placebo-controlled, clinical trial
Purpose:
To investigate the effects of silymarin on the liver in patients with liver disease.
Dose:
420 mg/day of silymarin or placebo
Participants:
97 male and female patients with liver disease with an average age of 35 years (treatment group) and 39 years (control group)
Duration:
4 weeks
Results:
The study found an association between silymarin treatment and significant reductions in liver enzyme levels, indicating improved liver function. Additionally, 7 out of 23 people treated with silymarin showed complete normalisation in a liver function test, compared to only 1 out of 38 in the placebo group. Microscopic examination also showed more improvements in the silymarin group. No side effects were reported. Overall, silymarin treatment appears to significantly improve liver function in patients with liver disease.
Year:
1982
Link:
Study 4 (Acne)
Study type:
Randomised, single-blind, prospective, placebo-controlled clinical trial
Purpose:
To investigate the effects of oral silymarin (the main active ingredient in milk thistle extracts), N-acetylcysteine, and selenium on acne.
Dose:
There were 4 treatment groups: 210 mg/day of silymarin (3 x 70 mg tablets), 1200 mg/day of N-acetylcysteine (2 x 600 mg tablets), 200 mcg/day of Selenium (2 x 100 mcg tablets) or placebo
Additional interventions:
Topical moisturising cream applied once daily at bedtime
Participants:
56 male and female patients aged 14-30 years with acne and 28 healthy males and females
Duration:
8 weeks
Results:
A statistically significant association was found between silymarin supplementation and a reduction in the number of acne lesions at week 6 and 8 of treatment (-53% reduction in lesion count), whereas there was a non-significant reduction in the number of lesions in the placebo group.
Year:
2012
Link:
Psyllium Husk Fibre Scientific Studies
We have summarised the most interesting studies of psyllium husk fibre related to diabetes and constipation.
Study 1 (Constipation)
Study type:
Randomised clinical trial (Uncontrolled)
Purpose:
To compare the effects of mixed fibre and psyllium on bowel symptoms in patients with chronic constipation.
Method of Evaluation:
Constipation was evaluated using a stool and symptom diary. A questionnaire was used to evaluate bowel satisfaction, feelings of satiety, fullness after meals, abdominal bloating and flatulence. Quality of life was self-assessed with the Patient Assessment of Constipation Quality of Life questionnaire.
Dose:
10 g/day of psyllium (2 x 5g supplements dissolved in 8 oz. of liquid after meals) or 10 g/day of mixed fibre (2 x 5g supplements dissolved in 8 oz. of liquid after meals)
Participants:
72 males and females aged 18-75 years
Duration:
4 weeks
Results:
The study found an association between 10g/day mixed fibre and 10g/day psyllium and improvements in constipation and quality of life. However, mixed fibre was more effective in relieving flatulence and bloating than psyllium.
Year:
2016
Link:
Study 2 (Constipation)
Study type:
Randomised, single-blinded, placebo-controlled trial
Purpose:
To assess the effects of adding psyllium to a normal diet among patients with type 2 diabetes and chronic constipation
Method of Evaluation:
A questionnaire was used to evaluate the effects on constipation.
Dose:
10 g/day of psyllium (4 cookies x 2.5 g of psyllium) or placebo
Participants:
51 males and females with type 2 diabetes
Duration:
12 weeks
Results:
Compared with the baseline and placebo groups, the study found an association between 10 g/day of psyllium and improvements in constipation symptoms, body weight, glucose, and lipid values. Researchers also observed a significant reduction of body weight (-2.0kg body weight and -0.8 kg/m2 BMI) after 12 weeks of psyllium therapy.
Year:
2018
Link:
Study 3 (Diabetes)
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To determine the effects of psyllium in addition to low-fat diet in type II diabetic patients
Method of Evaluation:
Plasma glucose, total serum cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides levels were measured.
Dose:
15 g/day of psyllium (3 x 5g Psyllium powder dissolved into 250 mL water) or placebo
Additional Interventions:
Dietary therapy based on approximately 25 kcal/kg per day of basal requirement, consisting of 40%-50% carbohydrates, 1.0 g/kg per day of proteins and polyunsaturated fat.
Participants:
123 men and women with type II diabetes
Duration:
12 weeks
Results:
The study showed that 15g/day of psyllium added to a low-fat diet was associated with a significant decrease in LDL-cholesterol (“bad” cholesterol), triglycerides, glucose levels, total serum cholesterol and total cholesterol levels in diabetic patients after 12 weeks. Note that diabetes often raises triglycerides and LDL levels and thus increases the risk of heart disease and stroke.
The psyllium treatment was also found to be safe and well-tolerated by the participants.
Year:
1998
Link:
PRODUCTS CONTAINING PSYLLIUM HUSK FIBRE
Psyllium Husk Tablets

ZMA Scientific Studies
We have summarised the most interesting studies on ZMA.
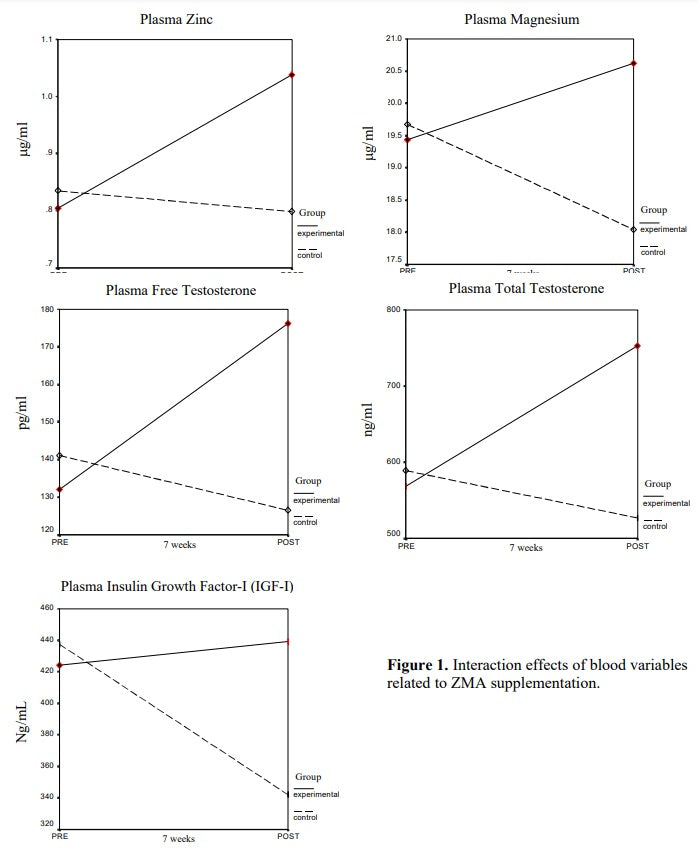
Study 1 (Testosterone)
Study type:
Randomised, double-blind trial
Purpose:
To evaluate the effects of zinc, magnesium aspartate, and vitamin B6 (ZMA) on the anabolic hormones and muscle function in varsity football players
Dose:
90 mg/day zinc monomethionine aspartate, 1350 mg/day magnesium aspartate, and 31.5 mg/day vitamin B-6 (3 x ZMA capsule with 30 mg zinc monomethionine aspartate, 450 mg magnesium aspartate, and 10.5 mg vitamin nightly between dinner and bedtime) or placebo
Additional interventions:
Training program such as spring football practice
Participants:
27 varsity football players
Duration:
7 weeks
Results:
The study found an association between ZMA (zinc, magnesium aspartate, and vitamin B6) intake during the athletes' training period and a significant increase in total testosterone, free testosterone, and Insulin-like growth factor I (IGF-1 is a hormone that helps promote bone and tissue growth). The researchers also observed increases in quadriceps torque by 10% and quadriceps power by 12.7%-15.2% in the group taking ZMA, which is significantly greater than the change of -0.8% to 2.4% in quadriceps torque and 8.6% to 10.8% change in quadriceps power in the placebo group.
Year:
2000
Link:
Creatine Scientific Studies
Several studies have investigated the effects of creatine. We have summarised the most interesting results.
Study 1 (Performance enhancement)
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To determine the effects of creatine on muscular strength, fat-free mass, resting metabolic rate, peripheral blood flow, and blood lipids
Method of evaluation:
Muscular strength was measured through one-repetition leg and bench press using standard methods.
Dose:
20 g/day of creatine monohydrate (4 x 5g creatine powder dissolved in flavoured dextrose drink mix) for the first 5 days and 10 g/day (2 x 5g creatine powder dissolved in flavoured dextrose drink) for the next 23 days or placebo
Additional Interventions:
3 days/week resistance training consisted of heavy resistance workouts using a combination of free weights and machines (Titan Exercise Equipment, Carrollton, TX) and were supervised by trained personnel.
Participants:
30 healthy active, but resistance-untrained, males with an average age of 21 years
Duration:
28 days
Results:
The study showed an association between 20 g/day of creatine intake and significant increases in body mass, body water, muscular strength, and resting metabolic rate after 28 days.
The addition of creatine supplementation to resistance training was associated with significant increases in total and fat-free body mass, muscular strength, peripheral blood flow (decreased peripheral blood flow may cause an injury to nerves and other tissues) and resting energy expenditure, as well as improvements in blood cholesterol.
Year:
2001
Link:
Study 2 (Performance enhancement)
Study type:
Meta-Analysis
Purpose:
To determine the effects of creatine in combination with resistance training on body composition, strength, and functional performance in older adults.
Intervention under study:
Creatine supplementation during a period of resistance training
Studies Reviewed
25 randomised, placebo-controlled trials
Results:
The results from the meta-analysis suggest that the combination of creatine supplementation and resistance training is associated with increased total body mass, fat-free mass, and increased leg and chest press one-rep max.
Year:
2013
Link:
Study 3 (Performance enhancement)
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To determine the effects of creatine supplementation on muscular strength and body mass during strength training.
Method of evaluation:
Isokinetic force was determined during a single squat movement by means of an isokinetic dynamometer.
Dose:
21 g/day of creatine monohydrate (7 g doses x 3 per day) for the first 5 days and 9 g/day (3 g doses x 3 per day) for the next 58 days or placebo
Additional interventions:
Strength-training program
Participants:
25 healthy male subjects with an average age of 22 years
Duration:
9 weeks
Results:
In participants who underwent both creatine supplementation and strength training, researchers observed a 2.9% significant increase in body mass. Meanwhile, no change in body mass was observed in the control and placebo groups. The study also showed that placebo and creatine groups had a similar increase in isokinetic force after 6 weeks of training whilst the control remained unchanged.
Year:
1999
Link:
PRODUCTS CONTAINING CREATINE
Creatine Monohydrate Powder

-
 Muscle GrowthMuscle Growth Rating 9/10.09/10
Muscle GrowthMuscle Growth Rating 9/10.09/10 -
 Strength GainsStrength Gains Rating 9/10.09/10
Strength GainsStrength Gains Rating 9/10.09/10
Collagen Scientific Studies
Several studies have investigated the effects of collagen. We have summarised the most interesting results.
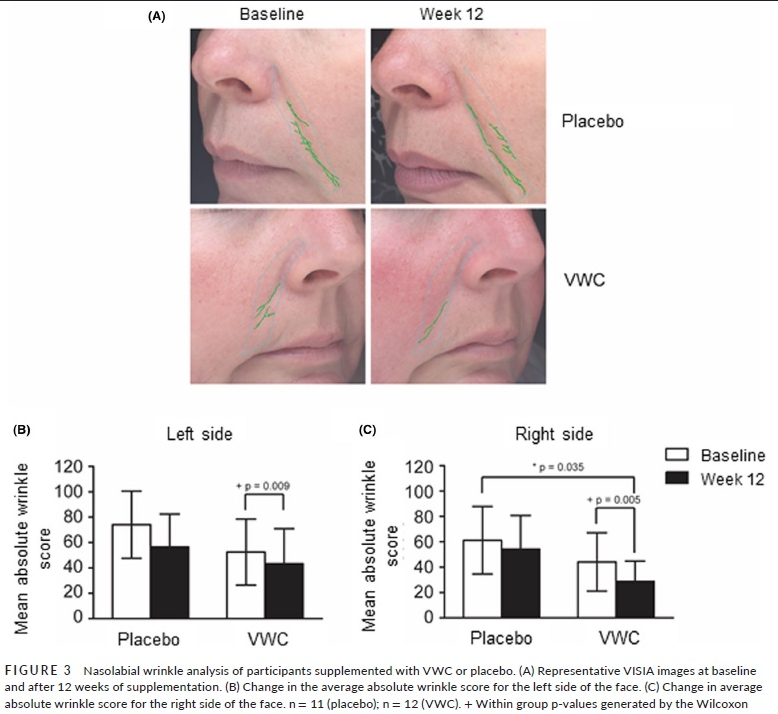
Study 1
Study type:
Randomised, triple-blind, placebo-controlled trial
Purpose:
To evaluate the clinical benefits of a fish‐derived collagen peptide on skin wrinkles and elasticity, and self‐reported skin appearance.
Method of evaluation:
Skin wrinkles were analysed using imaging to assess the number of wrinkles, damage and signs of ageing on and beneath the surface of the skin that is not visible to the human eye.
Skin elasticity measurements were performed using a Cutometer, a device that uses suction to measure elasticity.
Skin quality was self-assessed using a questionnaire. Participants were asked to report their skin health based on skin elasticity, hydration, radiance, firmness, wrinkles and overall feel.
Dose:
10 g/day of hydrolyzed collagen or placebo powder
Participants:
50 females aged 45-60 years
Duration:
12 weeks
Results:
The study found an association between collagen supplementation and significant reductions in face wrinkle scores compared to the placebo (lower scores are better). Also, participants taking collagen reported greater percentage improvements in overall skin appearance (9%) and wrinkles (15%), elasticity (23%), hydration (14%), radiance (22%), and firmness (25%) compared to the placebo group.
Year:
2021
Link:
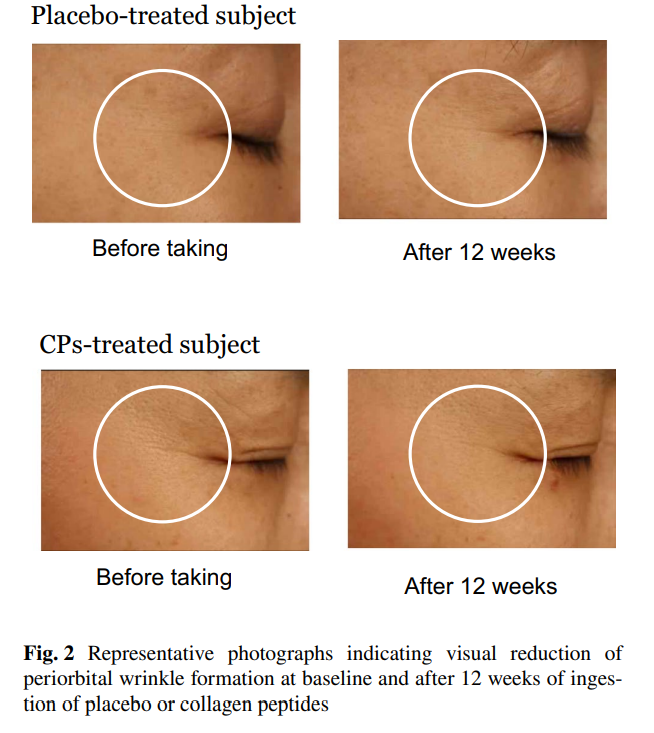
Study 2
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To evaluate the effects of fish scales-derived collagen peptides on periorbital wrinkles (wrinkles around the outer corners of the eyes), facial skin hydration, and skin elasticity in women.
Method of Evaluation:
Periorbital wrinkles were evaluated using a visiometer, which assesses wrinkles by analysing the light intensity passing through a silicone replica created from an impression moulded from the skin.
Skin moisture was measured with a device called a corneometer, which measures the moisture content of the outermost layer of the skin.
Skin elasticity measurements were performed using a Cutometer, a suction-based device that measures elasticity.
Dose:
3 g/day fish scales-derived of collagen peptides (in the form of 20 mL beverage) or placebo
Participants:
71 healthy women aged 30-60 years
Duration:
12 weeks
Results:
The study found an association between 12 weeks of collagen peptide oral intake and significant decreases in periorbital wrinkles compared to the control group. The researchers also observed a consistent trend of improved facial skin moisture and skin elasticity with collagen peptide supplementation, without any side effects or adverse events. These findings indicate that fish-derived collagen peptides hold great promise as a natural supplement for improving facial skin hydration, skin elasticity, and wrinkles.
Year:
2018
Link:
Study 3
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To evaluate the effects of collagen hydrolysate containing specific collagen peptides on parameters related to skin ageing
Method of Evaluation:
Skin elasticity measurements were performed using a Cutometer®, a device that uses suction to measure elasticity.
Skin hydration was assessed using the Corneometer, a device that measures skin hydration levels.
Transepidermal water loss, which is the evaporation rate of water through the outer layer of the skin, was assessed using the DermaLab® device. Transepidermal water loss levels can help assess the health of the skin barrier and can guide the selection of treatments to maintain or improve skin hydration and function.
Skin roughness was assessed by a 3D skin measurement device that measures skin roughness parameters.
Dose:
2.5 g/day or 5.0 g/day of collagen hydrolysate or placebo
Participants:
69 women aged 35-55 years
Duration:
8 weeks
Results:
Both dosages (2.5 and 5.0 g) of the collagen hydrolysate were both associated with statistically significant increases in skin elasticity after 4 and 8 weeks of daily consumption. It is important to note that skin elasticity is an important marker for skin ageing: as skin loses its elasticity, it starts to sag and wrinkle. In this study, skin elasticity increased up to 30% in some women after 8 weeks of treatment, and there was a statistically significant increase in skin elasticity among elderly women in the treatment group compared to the placebo. No significant effects were observed in the skin moisture and evaporation between the treatment group and the placebo group.
Year:
2014
Link:
Study 4
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To investigate the effects of collagen peptide supplementation on human skin hydration, wrinkling, and elasticity.
Method of Evaluation:
Skin hydration was measured with a device called a corneometer, which measures the hydration level of the outermost layer of the skin.
Skin wrinkling was assessed by visual assessment by dermatologists and a device used to measure the depth, length, and other characteristics of the wrinkles.
Skin elasticity measurements were performed using a Cutometer, a suction-based device that measures elasticity.
Dose:
1000 mg/day of low-molecular-weight collagen peptide or placebo
Participants:
64 women aged 40-60 years
Duration:
12 weeks
Results:
The study found an association between oral ingestion of 1000 mg of collagen once daily and significant improvements in skin hydration after 6 weeks and 12 weeks compared to the placebo group. The increase in skin hydration in the collagen group was 7.23-fold greater than in the placebo group at 6 weeks, and 2.9-fold greater at 12 weeks In addition,significant improvements in skin wrinkling and elasticity after 12 weeks was observed. None of these parameters were significantly improved in the placebo group.
The collagen was well tolerated by the participants. No adverse reactions were observed during the course of the study.
Year:
2018
Link:
Study 5
Study type:
Randomised controlled trial
Purpose:
To assess the effects of a hydrolyzed collagen supplement on skin moisturization, smoothness, and wrinkles.
Method of Evaluation:
Skin moisture was measured with a device called a corneometer, which measures the moisture content of the outermost layer of the skin.
Skin elasticity measurements were performed using a Cutometer, a suction-based device that measures elasticity.
Wrinkle depth measurements were taken using an Antera 3D, a 3D imaging device that takes images of areas of the face of study participants and analyses wrinkle depth using the software.
Clinical evaluations were performed by a dermatologist through visual assessment which evaluates skin softness, skin firmness, skin smoothness, and visibility of wrinkles.
Dose:
1st month: 1g/10 kg of body weight/day (dissolved in water once a day in the morning, during or immediately after breakfast) or placebo.
2nd month: 5 g/day of hydrolyzed collagen (dissolved in water once a day in the morning, during or immediately after breakfast) or control (maltodextrin)
Participants:
52 healthy females aged 40-60 years
Duration:
56 days
Results:
The study found an association between hydrolyzed collagen treatment and significant improvements on skin health over 28 and 56 days. The researchers observed improved skin moisturization and a progressive decrease in wrinkle depth, compared to baseline. The skin elasticity of the participants taking the hydrolyzed collagen supplement also increased significantly, which is considered a desirable outcome as it helps reduce wrinkles and gives the skin a more youthful appearance.
Additional clinical evaluations conducted by a dermatologist provided further support for these findings. After 28 and 56 days of treatment, participants in the collagen group showed significant improvements in skin softness, smoothness, firmness, and reduced visibility of wrinkles. Specifically, skin softness significantly improved in 35% of collagen group participants after 28 days, and increased to 54% after 56 days of treatment. Skin smoothness and skin firmness also showed statistical improvements in 27% of participants after 28 days and nearly reached a two-fold increase after 56 days (46% and 58% respectively). Lastly, wrinkle visibility decreased in 38% of collagen group participants after 56 days of treatment. These findings suggest that hydrolyzed collagen supplements may be an effective dietary supplement for improving skin health and reducing the signs of ageing.
Year:
2022
Link:
Study 6
Study type:
Randomised, double-blind placebo-controlled trial
Purpose:
To evaluate the effect of oral collagen hydrolysate on skin elasticity (the skin's ability to stretch and return to its original form without permanently changing shape or tearing) and compare its effects on sun-exposed and sun-protected areas of the skin. Sun exposure is a major factor that can affect skin elasticity negatively.
Method of Evaluation:
Skin elasticity measurements were performed using a Cutometer, a suction-based device that measures elasticity.
Dose:
5g/day of collagen hydrolysate powder (dissolved in water and consumed in the morning before breakfast) or placebo
Participants:
36 women aged 50-60 years
Duration:
8 weeks (4 weeks treatment plus 4 weeks washout period)
Results:
The participants in the collagen hydrolysate group exhibited significant improvements in skin elasticity in both sun-exposed and sun-protected areas when compared to their baseline measurements, suggesting a positive impact on overall skin elasticity. When comparing the collagen hydrolysate group to the placebo group, significant improvements in skin elasticity were specifically observed in sun-exposed areas, indicating that collagen hydrolysate may have a greater effect on skin elasticity in areas exposed to the sun. It's noteworthy that the improvements in skin elasticity were still observed four weeks after discontinuation of the collagen hydrolysate supplementation. This suggests that the benefits of collagen hydrolysate on skin elasticity may have a lasting effect. No adverse events, such as nausea, vomiting, diarrhoea, or constipation, were reported in either group.
Overall, these findings suggest that daily intake of a 5-gram oral collagen hydrolysate may be a beneficial supplement for improving skin elasticity, particularly in sun-exposed areas.
Year:
2021
Link:
Study 7
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To investigate the effect of collagen tripeptide from fish skin on skin hydration in middle-aged women after weather condition adjustments. The researchers aimed to adjust for weather conditions, including temperature, humidity, and ultraviolet A exposure, to provide a more accurate evaluation of the effects of collagen tripeptide on skin hydration, as these factors have been shown to impact skin barrier function and accelerate water loss, resulting in dryness and dermatitis.
Method of evaluation:
Skin hydration was assessed using the Corneometer, a device that measures skin hydration levels.
Skin elasticity measurements were performed using a Cutometer, a suction-based device that measures elasticity.
Skin wrinkling was evaluated with a device called a skin visiometer, a device that uses imaging technology, such as high-resolution cameras and specialised software, to capture and analyse the surface of the skin.
Skin transepidermal water loss measurements were performed using a device called an evaporimeter, which measures transepidermal water loss by assessing the rate at which water evaporates from the skin into a controlled environment, providing insights into skin barrier function and hydration.
Subjective improvement in skin properties were assessed using self-reported questionnaires which measured the participants perception of improvement in skin properties with time.
Dose:
1000 mg/day of collagen tripeptides (4 x 250 mg capsules) or placebo
Participants:
84 women aged 40-60 years (74 women completed the trial)
Duration:
12 weeks
Results:
The study found an association between the oral intake of 1,000 mg of collagen tripeptides for 12 weeks and a significant decrease in transepidermal water loss, even after adjusting for weather conditions in the region (humidity, temperature, and ultraviolet A exposure) compared to placebo. A reduction in transepidermal water loss indicates a decrease in the amount of water that evaporates from the skin's surface, signifying improved skin barrier function and increased moisture retention, which is generally considered beneficial for skin health and hydration. In terms of skin hydration, more improvement was evident in the collagen tripeptide group than in the control group, although there was no statistical significance in the differences in change from the start of the study between the two groups.
Subjects under 50 years of age reported significant improvements in the appearance of their skin and moisture levels after taking collagen tripeptide for 12 weeks, while subjects over 50 years of age reported significant improvements in skin tightness compared to the control group after 6 weeks of collagen tripeptide supplementation. Tighter skin often appears more youthful and less saggy. Furthermore, collagen tripeptide was found to be safe and well-tolerated among middle-aged women.
Overall, the study findings suggest that collagen tripeptide may be effective in reducing water loss in middle-aged women.
Year:
2020
Link:
Study 8
Study type:
Randomised, double-blind, placebo-controlled, parallel study
Purpose:
To investigate the effects of an oral liquid supplement containing both collagen bioactive peptides and antioxidants in skin texture and properties.
Method of evaluation:
Skin elasticity measurements were performed using a SkinLab USB Elasticity Module, a skin analysis device that measures elasticity.
Skin structures were examined through skin biopsies and microscopic examinations to assess skin structures and, in particular, of collagen and elastin fibres.
Self-assessment questionnaires consisted of questions related to skin, hair, nails, joints, mood, and photo-ageing conditions.
Dose:
5,000 mg/bottle/day of hydrolyzed collagen type I in a beverage drink or placebo
Participants:
120 male and female volunteers aged 40-60 years with and without cosmetic surgery
Duration:
90 days
Results:
The researchers observed a significant 7.5% increase in participants' skin elasticity after 90 days of daily consumption of the test beverage containing 5,000 mg of hydrolyzed collagen compared to baseline. This increase in skin elasticity was observed in participants who had undergone cosmetic treatment and participants who had not, with significantly greater effects in individuals who did not undergo cosmetic treatment (9.1% vs. 13.9%). In the placebo group, a significant 5% decrease from the start of the study in participants' skin elasticity was observed. However, no significant difference in skin elasticity between the test product and placebo on day 0 and day 90 was found.
In addition, microscopic skin examinations conducted on two participants found improvements in the structure and layering of the outermost skin layers and in the network of collagen and elastin fibres (proteins in the skin) after 90 days of collagen intake. These results suggest that oral intake of collagen may help improve skin health by restoring the correct thickness of skin layers and the balance of collagen and elastin.
Moreover, results from the self-assessment questionnaires showed an overall significant perceived improvement in skin, hair and nail appearance and mood at the end of the treatment. These improvements were not observed to the same extent in the placebo group. The perception of skin at the end of the study was similar between participants who underwent a cosmetic procedure and participants who did not, indicating that the test product had consistent benefits.
No adverse events were reported in the study.
Year:
2017
Link:
Study 9
Study type:
Randomised, placebo-controlled trial
Purpose:
To investigate the effects of bioactive collagen hydrolysates on facial skin moisture, elasticity and facial ageing signs.
Method of evaluation:
Skin moisture was measured with a device called a corneometer, which measures the moisture content of the outermost layer of the skin.
Skin elasticity measurements were performed using a Cutometer, a device that uses suction to measure elasticity.
Skin wrinkles and roughness were evaluated with a device called a VisioFace, a skin surface analysis device which measures the number of wrinkles, wrinkle area, wrinkle depth, and roughness.
Dose:
0.5 g/day or 10 g/day of bioactive collagen peptides or placebo
Participants:
85 females aged 35 to 55 years (80 completed the study)
Duration:
8 weeks
Results:
Participants who took collagen hydrolysate with a higher content of bioactive collagen peptides showed significantly greater improvement in terms of facial skin moisture, elasticity, wrinkles, and roughness compared to those who took collagen hydrolysate with a lower content of bioactive collagen peptides or the placebo group. These improvements are beneficial as they enhance overall appearance, confidence, and comfort while contributing to the long-term health and resilience of the skin. No adverse events were reported during the trial.
Year:
2015
Link:
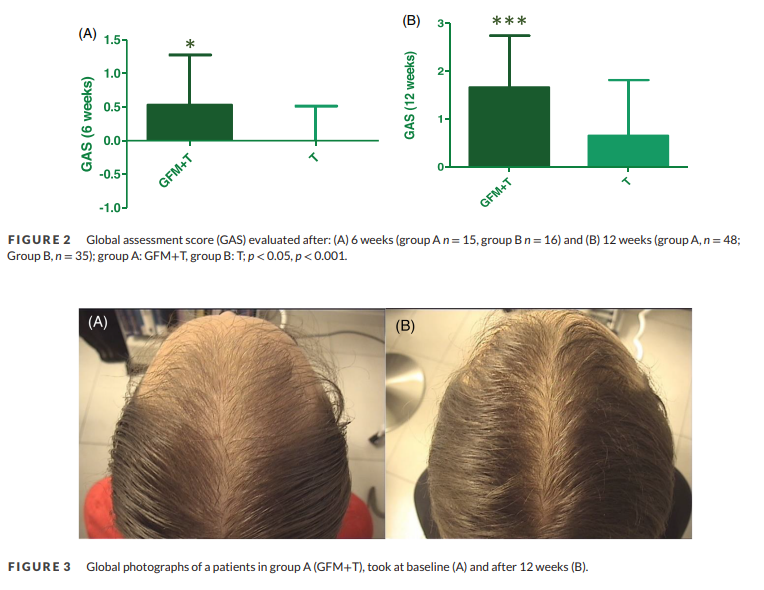
Study 1
Study type:
Prospective, randomised, assessor-blinded, controlled trial
Purpose:
To investigate the effects of an oral supplement containing marine hydrolyzed collagen, amino acids (building blocks of protein), and micronutrients in subjects with hair loss.
Method of Evaluation:
Improvements in hair loss were evaluated by a panel of six dermatologists using a rating scale called global assessment score, which measures overall improvement over time from −3 (severe worsening) to +3 (excellent improvement). Higher scores signify a more positive outcome or greater improvement.
Dose:
300 mg/day of hydrolysed fish-origin collagen (in a tablet formulation along with taurine, cysteine, methionine, iron, and selenium) or control (drug treatment alone)
Additional interventions:
A specific drug treatment decided by the investigator according to the type of
hair loss
Participants:
83 men and women with an average age of 41 years (76 completed the trial)
Duration:
12 weeks
Results:
The researchers observed significant improvements in hair loss after 12 weeks of oral supplementation with hydrolyzed fish-origin collagen (combined with a specific drug treatment) when compared to drug treatment alone. The researchers also observed a higher percentage of participants in the oral supplement group (50%) exhibiting moderate improvements compared to the control group (23%). Both the oral supplement and the drug treatments were generally well tolerated, as reported by investigators and patients. Overall, the results suggest that the tested oral supplement, containing hydrolysed fish-origin collagen, taurine, cysteine, methionine, iron and selenium has the potential to improve the effects of specific anti-hair loss drug treatments in subjects with hair loss disorders.
Year:
2023
Link:
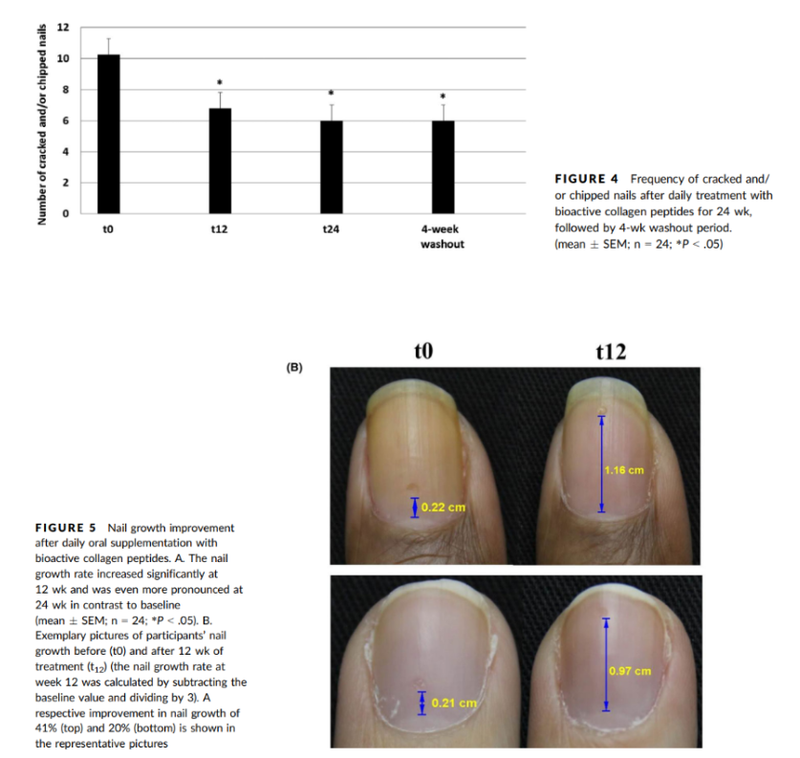
Study 2
Study type:
Open-label, uncontrolled clinical trial
Purpose:
To assess the effects of specific bioactive collagen peptides on nail growth and brittle nails in women with signs of brittle nails.
Method of Evaluation:
Brittle nail symptoms and overall improvement of the nails were evaluated by a dermatologist using a subjective 5-point scale, which measures symptoms like nail peeling, edge irregularities, and nail roughness.
Frequency of cracked and/or chipped nails were recorded daily by the participants, specifying how many times their nails had been cracked or chipped on each hand.
Nail growth was evaluated by measuring the distance from the edge of the lunula, the crescent-shaped, white area at the base of the nail, to the point marked before baseline.
Patient overall improvement was assessed using a self-reported questionnaire and rating scales.
Dose:
2.5 g of bioactive collagen peptides (dissolved preferably in water)
Participants:
25 healthy women aged 18-50 years (24 participants completed the trial and were included in the analysis)
Duration:
24 weeks of treatment
Results:
The researchers observed a significant increase in the average nail growth rate (12%) and a significant reduction in the average frequency of broken nails (42%) after 24 weeks of collagen treatment compared to baseline. Additionally, 64% of participants displayed clinical improvements in nail symptoms rated as excellent, good, or fair, and 88% exhibited similar improvements four weeks after the end of treatment. The majority of participants (80%) also reported that the use of bioactive collagen peptides improved the appearance of their nails and were completely satisfied with the performance of the bioactive collagen peptides treatment. Overall, the findings suggest that daily intake of bioactive collagen peptides may have the potential to increase nail growth and improve brittle nails, along with a notable decrease in the frequency of broken nails.
Year:
2017
Link:
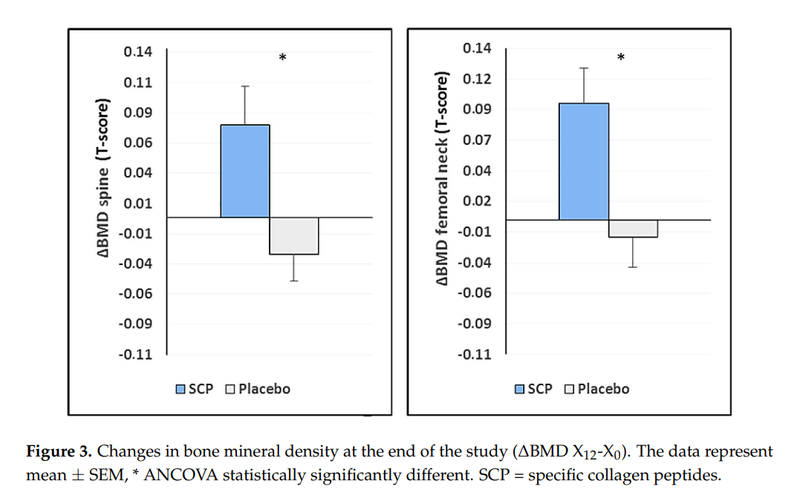
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To investigate the effect of specific collagen peptides in postmenopausal women with reduced bone mineral density, a measurement used to assess the amount calcium and other types of minerals in the bone. Bones with more minerals are denser, so they tend to be stronger and less likely to break.
Method of evaluation:
Bone mineral density was measured using a device called Dual-Energy X-ray Absorptiometry (DEXA), a medical imaging technique used to assess bone density. Specific biomarkers for bone formation and degradation were analysed through blood sample analysis.
Dose:
5 g/day of specific collagen peptides or placebo
Participants:
131 postmenopausal women with an average age of 64 years
Duration:
12 months
Results:
The researchers observed that bone mineral density significantly increased by almost 3.0% in the spine and 6.7% in the femoral neck (the region just below the ball of the hip joint) in the collagen group after daily oral administration of 5 g of collagen peptides for 12 months. Increased bone density means having denser and stronger bones, which reduces the risk of fractures. In contrast, the placebo group showed decreased bone mineral density (-1.3% for the spine and -1.0% in the femoral neck). It is important to note that the spine and femoral neck are critical weight-bearing areas, so assessing bone density in these regions provides valuable information about a person's overall bone health.
Additionally, the researchers observed a favourable change in bone markers in participants taking collagen. A marker of bone formation called “amino-terminal propeptide of type I collagen” (P1NP) significantly increased in the collagen group. This increase in bone formation is important for postmenopausal women as they are at a higher risk of osteoporosis, a condition characterised by low bone density and an increased risk of fractures. In contrast, a marker of bone degradation called “C-telopeptide of type I collagen” (CTX-1) significantly increased in the placebo group, which typically indicates an increase in bone resorption, which means that more bone is being broken down than normal, potentially weakening bones and leading to conditions like osteoporosis. No changes in bone degradation markers were observed in the collagen group.
Year:
2018
Link:
Study 2
Study type:
Randomised, parallel, double-blind clinical trial
Purpose:
To compare the effectiveness and safety of oral hydrolysed collagen versus glucosamine sulphate (a widely used supplement particularly for individuals with osteoarthritis) in patients with early stages of knee osteoarthritis.
Method of Evaluation:
A scale of 0 to 10 was used to assess joint pain at its worst, at its best, at that moment in time and on average.
Overall health status was assessed using a self-reported questionnaire that assesses pain, stiffness and physical function in patients with knee osteoarthritis.
Quality of life was also assessed using a self-reported questionnaire, which measured aspects such as general health, vitality, and physical and social function.
Dose:
10 g/day of enzymatic hydrolysed collagen or 1.5 g/day of glucosamine sulphate
Participants:
100 men and women aged 40 years and older with knee osteoarthritis (93 completed the trial)
Duration:
90 days
Results:
The researchers observed significantly reduced pain intensity in both the hydrolyzed collagen and glucosamine sulphate groups (a common dietary supplement for osteoarthritis). However, greater effects were observed in the hydrolyzed collagen group, with 68% of patients demonstrating a clear improvement in pain assessment, compared to 37% of patients treated with glucosamine sulphate. A higher percentage of patients treated with hydrolyzed collagen (34.8%) also reported improved health status in terms of pain, stiffness, and physical function, compared to those treated with glucosamine sulphate (13.3%), and this difference was statistically significant.
Additionally, the hydrolyzed collagen group reported better improvements in quality of life than the glucosamine sulphate group. Moreover, the subjective opinions of both the investigators and patients indicated that hydrolysed collagen has better effects than glucosamine sulphate.
The incidence of adverse events was similar in both groups, and both hydrolysed collagen and glucosamine sulphate were well tolerated.
Year:
2011
Link:
PRODUCTS CONTAINING COLLAGEN
Collagen Powder

NMN, Resveratrol & Collagen Bundle

RESVERATROL SCIENTIFIC STUDIES
We have summarised the most interesting findings from several studies that have investigated the effects of resveratrol.
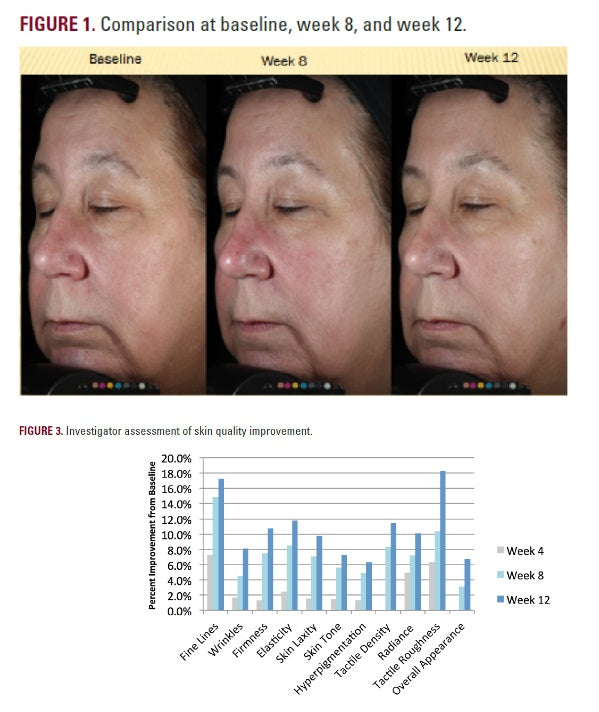
Study 1
Study type:
Clinical trial
Purpose:
To assess the effectiveness and tolerance of a nighttime topical antioxidant formulation containing resveratrol, baicalin, and vitamin E for treating mild to moderately photodamaged skin. Photodamaged skin refers to skin damage caused by excessive sun exposure over time.
Method of evaluation:
The efficacy of the treatment was evaluated by assessing improvements in the skin's appearance, texture, and overall photodamage.
Dose:
Participants applied a topical resveratrol formulation to their skin before bedtime. The formulation consisted of 1% resveratrol, 0.5% baicalin, and 1% vitamin E.
Participants:
55 healthy females aged 40 to 60
Duration:
12 weeks
Results:
The study found that both baicalin and resveratrol can penetrate the outermost layer of the skin, known as the stratum corneum, and reach deeper layers. The deeper cutaneous layers, such as the dermis and epidermis, contain various cells and structures that are involved in skin ageing and damages. By reaching these layers, the ingredients can exert their antioxidant, anti-inflammatory, and collagen-stimulating effects, which can improve skin texture, firmness, elasticity, and pigmentation. The researchers also observed significant improvements in various skin parameters, including fine lines, wrinkles, firmness, elasticity, skin laxity, skin tone, hyperpigmentation, radiance, tactile roughness, and pinch recoil measurements (a type of skin elasticity test) in the crow's feet area—the fine lines and wrinkles at the outer corners of the eyes. These improvements were observed at weeks 4, 8, and 12 of using the topical formulation. Additionally, improvements were also noted in skin density and overall appearance at weeks 8 and 12 of using the formulation.
Year:
2011
Link:
Study 2
Study type:
Cellular study (in vitro) and human experiments (in vivo)
Purpose:
To evaluate whether resveratrol could provide antioxidant benefits and if it could effectively penetrate skin when applied topically.
Dose:
10 μL of 5% resveratrol solution was applied to each square centimetre of skin (10 μL per square centimetre is about the size of a droplet or tiny dot on skin).
Participants:
6 women with no history of dermatological disease
Duration:
24 hours application time
Results:
The study found an association between topical treatment with resveratrol and higher antioxidant effects in the deeper layers of the stratum corneum, which is the outermost layer of skin. The researchers also observed significant concentrations of resveratrol in different layers of the stratum corneum. The retention of active resveratrol and its high antioxidant effects within the stratum corneum suggests its potential as an effective treatment for safeguarding the skin's surface against damage caused by free radicals and environmental aggressors such as sun radiation and pollution.
Year:
2016
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled cross-over study
Purpose:
To investigate the anti-inflammatory and antioxidant effects of resveratrol on healthy smokers.
Dose:
500 mg/day of resveratrol (tablet) or placebo
Participants:
49 male and female healthy smokers with an average age of 35
Duration:
30 days of resveratrol and 30 days of placebo
Results:
The researchers observed a reduction of approximately 50% in C-reactive protein concentrations after one month of resveratrol supplementation. A reduction in C-reactive protein concentration is generally considered good because it indicates a decrease in inflammation in the body. C-reactive protein is a protein produced by the liver in response to inflammation, infection, or tissue injury. It is often used as a marker of systemic inflammation in medical tests. The study also found an association between resveratrol supplementation and a decrease in triglyceride concentrations, as well as an increase in total antioxidant status. Maintaining lower levels of triglycerides is important for promoting cardiovascular health, metabolic health, pancreatic function, and liver health.
No adverse events were reported in either group after supplementation.
Year:
2013
Link:
Study 1
Study type:
Meta-analysis of randomised controlled trials
Purpose:
To explore the effects of resveratrol supplementation on participants’ blood lipid profile (including substances like triglycerides (fats), cholesterol, and phospholipids). Blood lipid analyses play a crucial role in assessing cardiovascular health and guiding interventions to manage dyslipidemia, a medical condition characterised by abnormally high blood lipid concentrations, which is a major risk factor for cardiovascular disease.
Dosages:
Resveratrol intervention doses ranged from 5 to 3000 mg/day and intake varied from 4 to 48 weeks
Number of Studies Reviewed:
17 studies were included in this meta-analysis
Results:
The findings from the review showed that the intake of resveratrol could significantly decrease the total cholesterol, triglyceride (a type of fat in the blood). A reduction in these levels can help improve overall cardiovascular health and lower the risk of heart-related problems.
In addition, low-density lipoprotein cholesterol (“bad” cholesterol) was also found to be reduced by resveratrol supplementation. LDL-cholesterol is often referred to as "bad" cholesterol because high levels of it are associated with an increased risk of cardiovascular diseases, such as heart disease and stroke. Therefore, a lower LDL-cholesterol level is generally considered beneficial for heart health. The reduction of LDL-cholesterol induced by resveratrol was reported to be associated with the antioxidant effect of resveratrol. On the other hand, resveratrol did not alter the level of high-density lipoprotein cholesterol (often referred to as 'good' cholesterol).
Further analysis showed that the reduction of LDL-cholesterol was more significant in trials with a duration of ≥12 weeks and in subjects with type 2 diabetes mellitus. In addition, the analysis revealed that a high dosage of resveratrol, specifically 500 mg/d or more, showed an opposite effect size. This means that, in contrast to the reduced LDL-cholesterol levels observed with a low dosage, a high dosage of resveratrol increased LDL-cholesterol levels. The authors suggest that the dosage of resveratrol intervention is an essential factor that affects the level of LDL-cholesterol and should be considered when designing interventions to reduce LDL-cholesterol levels in individuals with high cholesterol or at risk of heart disease.
Year:
2022
Link:
Study 2
Study type:
Pilot study (uncontrolled)
Purpose:
To investigate the effects of resveratrol in older adults with impaired glucose tolerance, a medical condition in which an individual's blood sugar levels are higher than normal but not high enough to be classified as diabetes. People with impaired glucose tolerance are at an increased risk of developing type 2 diabetes, as their bodies struggle to regulate blood sugar effectively.
Dose:
1, 1.5, or 2 g/day of resveratrol
Participants:
10 men and women with an average age of 72 years, diagnosed with impaired glucose tolerance
Duration:
4 weeks
Results:
The researchers observed that the fasting blood sugar of participants taking resveratrol for 4 weeks did not change. However, their peak sugar levels after meals, as well as the total amount of blood sugar over the span of 3 hours, decreased significantly. This means that after taking resveratrol, the participants' blood sugar didn't spike as much as it usually would after eating, which is a positive sign for better sugar control. Normally, blood sugar rises after a meal and then gradually decreases as the body's cells use or store the sugar for energy. However, if sugar levels remain elevated several hours after eating, it can indicate insulin resistance, a condition where cells struggle to efficiently absorb sugar from the blood.
Furthermore, the researchers observed improved insulin sensitivity after taking resveratrol. This suggests that cells in the body are becoming more efficient at utilising insulin to regulate blood sugar levels. The researchers also observed a trend towards an improved post-meal reactive hyperemia index, which is a measure of the functioning of the inner lining of blood vessels that plays a crucial role in regulating various cardiovascular functions and maintaining overall health of blood vessels.
These findings suggest that resveratrol may have a positive effect on post-meal sugar levels, overall sugar metabolism, and vascular function (blood vessels) in older adults with impaired glucose tolerance.
Year:
2012
Link:
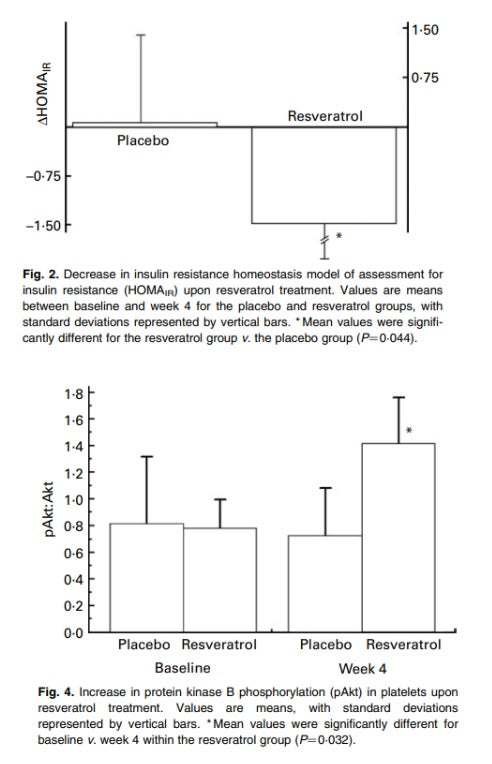
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To determine the effects of resveratrol on insulin sensitivity and oxidative stress among patients with type 2 diabetes
Dose:
10 mg/day of resveratrol (2 x 5 mg capsules) or placebo
Participants:
19 males previously diagnosed with type 2 diabetes.
Duration:
4 weeks
Results:
The study found an association between 5 mg/day of resveratrol and a significant decrease in insulin resistance. Insulin resistance is a condition where the body's cells become resistant to the effects of insulin, leading to high blood sugar levels, and it is a characteristic feature of type 2 diabetes. The researchers also observed increases in the ratio of phosphorylated protein kinase B (pAkt):protein kinase B (Akt) in platelets (blood-clotting cells) after 4 weeks of resveratrol treatment. This ratio is often used as a measure of Akt pathway activation in cells. The Akt pathway is involved in various cellular processes, including cell survival, growth, and metabolism.
Year:
2011
Link:
Study 1
Study type:
Three-arm, two-site pilot, randomised, controlled trial
Purpose:
To evaluate the safety and effects of resveratrol in combination with exercise in older adults with physical function limitations.
Dose:
500mg/day of resveratrol (1 x 500 mg capsules; 1 x 500 mg placebo capsules) or 1000 mg/day (2 x 500 mg capsules) of resveratrol or placebo
Additional interventions:
Supervised walking and whole-body resistance exercise training program performed twice a week.
Participants:
60 older adults aged 65 years and older. A total of 85% of the participants complied with the resveratrol regimen as instructed.
Duration:
12 weeks
Results:
In terms of physical performance, the study found an association between exercise combined with 1,000 mg/day resveratrol supplementation and improved physical and mitochondrial function in older adults. Mitochondrial function involves processes within cells that maintain energy balance and plays a vital role in physical function, especially in the context of exercise and overall fitness.
The researchers observed a relatively low number of possibly related adverse events to the trial. The most common issues were gastrointestinal-related (9 participants) and dizziness. Of these, 2 were in the 1000 mg/day group and 5 were in the 500 mg/day group. Two serious adverse events were reported, but they were found to be unrelated to the trial.
Overall, the findings suggest that resveratrol supplementation at doses up to 1000 mg/day have potential benefits for both physical and mitochondrial function in older adults, with low number of related, or possibly related adverse events.
Year:
2020
Link:
Study 2
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To evaluate the effects of short-term resveratrol supplementation on metabolic and safety outcomes in generally healthy overweight, older adults.
Dose:
300 or 1000 mg/day (2 x 150 or 500 mg capsules) of resveratrol or placebo
Participants:
32 overweight adult men and women aged 65 years and older
Duration:
90 days
Results:
The researchers observed that adverse reactions were consistently low in all groups, including the placebo group. Diarrhoea was the most frequently reported adverse reaction in the 300 mg/day group (33%), and two participants in the 1000 mg/day group withdrew from the study due to gastrointestinal issues. However, there were no statistically significant differences in reported adverse reactions between participants in the treatment group and placebo group.
In addition, the researchers also observed that blood sugar levels remained stable among participants in both of the resveratrol groups. In contrast, participants receiving the placebo experienced a significant increase in blood sugar levels compared to their initial levels over the course of the study.
No significant changes in blood pressure, body weight, or waist circumference were observed in any group.
Year:
2014
Link:
Study 3
Study type:
Clinical trial (uncontrolled)
Purpose:
To explore the safety and the effect of resveratrol on the insulin-like growth factor axis, which is a complex hormonal system involving proteins and factors that play a crucial role in regulating growth and development in the body.
Dose:
0.5, 1.0, 2.5, or 5.0 g of resveratrol (1, 2, 5 or 10 x 500 mg caplets)
Participants:
40 male and female healthy volunteers with an average age of 35-42 years
Duration:
29 days
Results:
The researchers observed that resveratrol was safe, with no serious adverse reactions observed through clinical or biochemical blood tests during the study period and follow-up. Among the 40 participants, some experienced adverse effects, with gastrointestinal symptoms like nausea, flatulence, abdominal discomfort, and diarrhoea being the most common, especially at higher doses (2.5 g and 5.0 g). Most of these events were mild, but some participants at the higher doses experienced moderate symptoms.
The symptoms, which typically got better during the day but returned after the next dose, was resolved within just 2 days after finishing the 29-day course. No weight loss was observed in any participant, and the treatment did not cause any significant impairment in the participants' ability to carry out their daily activities throughout the study period.
On the basis of these findings, the researchers recommended that in future intervention studies of resveratrol the daily dose should perhaps not exceed 1.0 g.
Year:
2010
Link:
Study 4
Study type:
Open-label clinical trial (uncontrolled)
Purpose:
To assess the pharmacokinetic properties (how the body interacts with a drug after it has been administered) and safety of resveratrol following a 500 mg single oral dose.
Dose:
500 mg of resveratrol tablets
Participants:
15 healthy male and female volunteers, aged 18-55 years
Duration:
Single dose
Results:
No adverse reactions associated with resveratrol were reported during the study. The only moderate-intensity incident observed was a traumatic cutaneous wound, a skin injury resulting from physical trauma like cuts, scrapes, or scratches. This occurred in only one subject in the present study and was not linked to the resveratrol treatment used. Overall, the researchers observed that resveratrol tablets (500mg) were well-tolerated by all participants of the study.
Year:
2016
Link:
Study 5
Study type:
Rodent studies
Purpose:
To evaluate the potential toxicity of resveratrol at different dosages in rats.
Dose:
300, 1000, or 3000 mg/kg body weight/day of trans-resveratrol
Duration:
4 weeks
Results:
Rats administered with 3000 mg/kg body weight per day of resveratrol experienced adverse effects such as increased clinical signs of toxicity, reduced body weights and food consumption. The researchers also observed elevated levels of blood urea nitrogen, creatinine, alkaline phosphatase, alanine aminotransferase, total bilirubin, and albumin, which can suggest problems in kidney and liver issues. There was also reduction in the haemoglobin, hematocrit, and red cell counts, and increased white cell count, which could suggest that the rats' blood and immune systems were affected by the resveratrol treatment, potentially causing anaemia and altering their ability to fight infection. They also exhibited increased kidney weights and clinically significant kidney lesions (damaged kidneys). No histological effects on the liver were observed despite the clinical chemistry changes and increased liver weights in females.
Effects seen in the rats administered with 1000 mg/kg/day of resveratrol included reduced body weight gain (females only) and elevated white blood cell count (males only). White blood cells are a crucial component of the immune system, and an increase in their numbers often indicates that the body is responding to an infection, inflammation, or other health challenges.
No observed adverse effect level was 300 mg/kg body weight of resveratrol.
Year:
2004
Link:
PRODUCTS CONTAINING RESVERATROL
Resveratrol 98% Capsules

NMN, Resveratrol & Collagen Bundle

Lion's Mane Scientific Studies
Several studies have investigated the effects of lion's mane. We have compiled the most interesting results related to cognitive health, mood, anxiety, and sleep.
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To examine the effect of yamabushitake (Lion’s Mane) on patients with mild-cognitive impairments.
Method of evaluation:
Cognitive impairment was evaluated using the researchers’ self-developed cognitive assessment tool. It consisted of verbal questions and tasks that assess memory, attention, language, and visual-motor skills, providing a score that helps identify potential cognitive impairments.
Dose:
1000 mg/day (4 x 250 mg containing 96% lion’s mane) or placebo
Participants:
29 men and women aged 50 to 80 years old
Duration:
16 weeks
Results:
The study found an association between 1000mg of lion’s mane intake and a significant increase in cognitive function at weeks 8, 12, and 16 of the trial. The researchers also observed that the cognitive function of 71.4% of participants in the lion's mane group improved significantly, compared to only 6.6% in the placebo group. The cognitive function remained unchanged in the majority of the placebo group (86.7%) after 6 weeks, compared to only 1 participant (6.7%) in the Lion's mane group. No adverse effects of lion's mane were reported.
Year:
2009
Link:
Study 2
Study type:
Randomised, double-blind, placebo-controlled pilot trial
Purpose:
To investigate the effects of Hericium erinaceus (Lion’s mane) capsules on patients with mild Alzheimer’s Disease.
Method of Evaluation:
Treatment effects in patients with mild-to-moderate dementia were measured using four cognitive assessment questionnaires. Researchers also assessed participants' vision, as multiple studies have found a correlation between visual problems and various degrees of cognitive decline. Vision was assessed using a Pelli-Robson chart and a Snellen eye chart (a chart with different-sized letters that helps measure how well you can see from a distance). Researchers also used magnetic resonance imaging to evaluate brain white matter, as brain white matter plays a crucial role in brain function, and changes in white matter integrity (the condition of nerve fibres) have been associated with various cognitive disorders, including dementia. An increase in white matter fibre means that the brain's communication network is improving, which can lead to better cognitive abilities and a greater ability to learn and adapt.
Dose:
3 x 350 mg capsules (1,050 mg/day) of lion’s mane (each containing 5 mg/g of erinacine A) or placebo. Note that erinacine A is one of the key components of lion’s mane responsible for the neurotrophic effects (promoting the growth and survival potential of neurons) and neuroprotective effects (shielding neurons from damage or degeneration).
Participants:
41 male and female participants aged 50 years and above
Duration:
49 weeks
Results:
Participants taking lion’s mane experienced less cognitive decline and showed improvements in their ability to perceive clear outlines of small objects, known as contrast sensitivity. Reduced contrast sensitivity has been found to be associated with a higher risk of cognitive impairment. There were also positive improvements observed in cognitive assessment scores compared to baseline and placebo, but these effects did not reach statistical significance. Additionally, the total amount of white matter fibres showed a lesser decrease in the lion's mane group compared to the placebo group, although this finding did not reach statistical significance. Overall, lion's mane supplementation for 49 weeks achieved a better contrast sensitivity in patients with mild Alzheimer's disease.
Year:
2020
Link:
Study 3
Study type:
Randomised, double-blind, placebo-controlled study
Purpose:
To evaluate the effects of Lion’s mane supplementation on cognitive function.
Method of evaluation:
Cognitive function, impairment and memory were assessed and scored using a series of questions and tasks. Visual cognition was assessed by measuring the ability of participants to accurately recall and reproduce the details of presented drawings.
Dose:
3.2 g/day of lion’s mane powder (4 x 0.8 g supplements) or placebo. Specifically, the fruiting body of lion’s mane was used.
Participants:
31 healthy adults aged over 50 years
Duration:
12 weeks
Results:
The study found an association between oral lion's mane supplementation and improved cognitive function after 12 weeks of treatment. The researchers observed an increase in the questionnaire scores for cognitive ability in both the treatment and placebo groups, but only the lion's mane treatment group showed statistically significant improvement. An increase in the questionnaire score indicates an improvement in cognitive function. However, the study did not find significant differences in visual cognition and memory. Overall, the study contributes to the growing body of evidence supporting the potential cognitive benefits of lion's mane.
Year:
2019
Link:
Study 4
Study type:
Animal study
Purpose:
To assess the effects of lion's mane on brain ageing, learning, and memory in ageing mice.
Method of evaluation:
Learning and memory were assessed using mouse avoidance tests, which measured how well the mice could avoid unpleasant experiences and remember them for future reference.
Dose:
108, 215 and 431 mg/kg body weight/day of lion’s mane (enriched with erinacine-A) or control. Erinacine-A is a natural compound found in lion’s mane and is known for its potential to protect and regenerate nerve cells in the brain.
Duration:
12 weeks
Results:
Mice fed with lion's mane showed improved learning abilities and better memory retention based on their performance in the avoidance tests. Additionally, lion's mane supplementation significantly reduced levels of a marker of oxidative stress in the brain called TBARS (thiobarbituric acid reactive substances), with the highest dose having the most significant effect. Oxidative stress may occur when there are too many unstable molecules called free radicals in the body and not enough antioxidants to get rid of them, which can lead to cell damage. Reduced levels of TBARS typically indicate a lower level of oxidative damage to cells.
In male mice, the high dose of lion's mane also decreased cortical iNOS levels. Lower levels of iNOS may indicate a reduction in inflammation or oxidative stress in the cortical region of the brain, suggesting potential benefits. Moreover, the mice fed with lion's mane had a reduced number of amyloid-β peptide plaques in the brain, which are abnormal clumps of protein associated with Alzheimer's disease. These mice also had decreased levels of 8-OHdG, a marker of DNA damage. Elevated levels of 8-OHdG have been linked to the development or progression of Alzheimer's disease.
Based on these findings, the study suggests that long-term intake of lion's mane may decrease oxidative stress in the brain, prevent chronic inflammation, reduce amyloid aggregation, and ultimately improve learning and memory.
Year:
2021
Link:
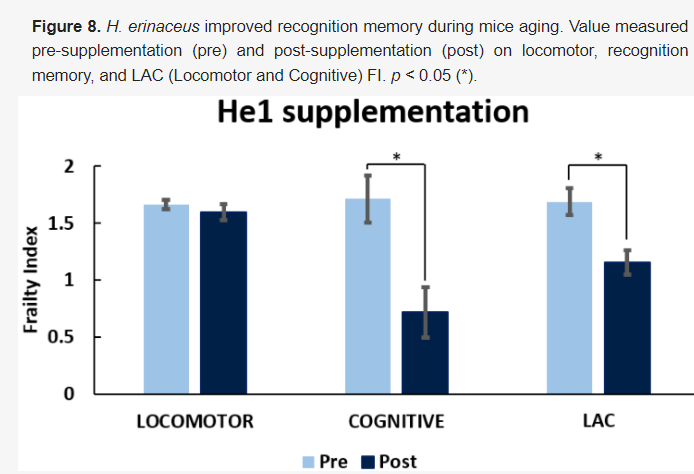
Study 5
Study type:
Animal study
Purpose:
To investigate the effects of lion’s mane on recognition memory and the growth of new nerve cells in the brain (neurogenesis) in ageing mice.
Dose:
1 g/day of lion’s mane
Duration:
2 months
Results:
Lion's mane supplementation was found to enhance recognition memory and, thus, reduce cognitive frailty in ageing mice.
Year:
2019
Link:
Study 1
Study type:
Pilot study (uncontrolled)
Purpose:
To investigate the effect of lion’s mane supplementation in overweight or obese individuals.
Method of evaluation:
Depression, anxiety, and binge eating disorders were assessed using self-reported questionnaires.
Dose:
1500 mg/day of lion’s mane (3 x 400 mg of lion’s mane mycelium + 100 mg of lion’s mane fruiting body extract)
Participants:
77 overweight or obese volunteers with an average age of 53 years
Duration:
8 weeks
Results:
The study found an association between 8 weeks of lion's mane supplementation and a significant decrease in depression, anxiety, and sleep disorders, along with an improvement in the quality of nighttime rest. When the results from the questionnaires were combined, the researchers observed an approximate 30% decrease in depression questionnaire scores and over 40% in anxiety symptoms. These findings suggest that lion's mane supplementation holds promise as a potential intervention for improving the mental well-being of overweight and obese individuals.
Year:
2019
Link:
Study 2
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To investigate the effects of Hericium erinaceus (Lion’s mane) on women with menopause, depression, sleep quality, and various complaints such as such as cognitive dysfunction, thinning of hair, low back pain, back pain, irritation, anxiety and apathy.
Method of Evaluation:
The participants were asked about the severity of their menopausal symptoms, such as depressive moods, vertigo, headache, heart palpitation, hot flashes, joint pain, loss of concentration, nervousness, excessive perspiration and sleep disturbances. Depressive symptoms and sleep quality were self-assessed using questionnaires. Participants were asked to rate their subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction.
Dose:
2 g/day of lion’s mane powder (4 cookies x 0.50 g of powdered lion’s mane fruiting bud) or placebo
Participants:
26 females with an average age of 41 years
Duration:
4 weeks
Results:
The researchers found an association between 4 weeks of lion's mane ingestion and significantly lower scores on questionnaires for depression and complaints such as cognitive dysfunction, thinning of hair, low back pain, back pain, irritation, anxiety and apathy. There was also a trend toward an improvement in sleep quality although the results were not statistically significant.
Year:
2010
Link:
Study 3
Study type:
Animal study
Purpose:
To investigate the effects of lion’s mane on sleep disruption and anxiety behaviour in mice.
Method of evaluation:
Sleep was measured using tests that measure electrical activity in the brain and muscles (electroencephalogram and electromyogram tests). These tests gather information about brain activity, muscle activity, and overall sleep quality. Anxiety behaviour was assessed using behavioural tests.
Dose:
75 and 150 mg/kg/day of lion’s mane mycelium
Additional intervention:
The mice were subjected to the tail suspension test for 15 min every day for 9 consecutive days to cause sleep disruption. In this test, the animal is suspended by its tail. Previous studies have shown that the tail suspension test is an acute stressor that causes sleep disruptions in mice. Lion’s mane was given to the mice 20 min prior to the tail suspension test.
Duration:
9 days
Results:
Both doses of lion's mane increased the duration of non-rapid eye movement (NREM) sleep during the dark period, indicating a greater amount of deep and restorative sleep. In the low-dose group (75 mg/kg), this effect occurred later in the dark period (between 7pm and midnight), while in the high-dose group (150 mg/kg), it was observed throughout most of the 12-hour dark period (between the hours 3pm and midnight).
In the behavioural tests, mice treated with the higher dose of lion’s mane exhibited a significant increase in exploration. This suggests that they had lower anxiety levels compared to the control group. Furthermore, there was a significant increase in dopamine levels, indicating positive effects on mood and motivation.
The study suggests that lion’s mane mycelium may potentially help to relieve anxiety by improving sleep disruptions and enhancing mood.
Year:
2021
Link:
Study 4
Study type:
Rodent study
Purpose:
To investigate the antidepressant-like effects of lion’s mane on stressed mice.
Method of evaluation:
The antidepressant-like behavioural responses were assessed using the mouse tail suspension test and forced swimming test. In the forced swimming test, the mice were placed in a water container where they could not escape. In the tail suspension test, mice are individually suspended by their tails using adhesive tape or other gentle means, allowing their bodies to hang freely in mid-air. The duration of immobility is measured in both tests as an indicator of the mice's level of despair or hopelessness. In these tests, mice exhibit behaviours such as immobility, struggle, or attempts to escape. It is believed that if the mice become more active, their mood and motivation has improved.
Emotional reactivity was assessed using an elevated plus maze, which evaluates the anxiety levels and emotional responses in mice. Briefly, the test takes advantage of the avoidance of rodents to open spaces, as well as their tendency to seek out enclosed and safer areas. By measuring the time spent in open versus enclosed areas, researchers can gain insights into an animal's anxiety levels and its preference for either risk-taking or risk-avoidance behaviours.
Dose:
100, 200 or 400 mg/kg body weight/day of lion’s mane mycelium. The lion’s mane was enriched with erinacine A, the primary active compound found in lion's mane extract that reportedly enhances the functioning of nerve cells in the brain and nervous system.
Duration:
4 weeks
Results:
Lion’s mane reversed depressive-like behaviour in mice. Researchers also observed increased levels of neurotransmitters (norepinephrine, dopamine, and serotonin) following lion's mane supplementation. These neurotransmitters are involved in mood regulation, and increased levels suggest potential enhancements in brain activity related to arousal, motivation, reward processing, positive emotions, and mood. There was also a reduction in pro-inflammatory cytokines in the mice, which indicates a decrease in inflammation. Cytokines are small proteins produced by the immune system that help coordinate the body's response to infections and inflammation.
Overall, the study indicates that lion's mane mycelium enriched with erinacine A shows promise as a potential treatment for depressive disorders.
Year:
2018
Link:
PRODUCTS CONTAINING LION'S MANE
Lion’s Mane Capsules

Lion’s Mane Powder

Turkey Tail Scientific Studies
The effects of turkey tail have been extensively investigated through numerous clinical trials and rodent studies. We have summarised some of the most interesting scientific studies related to immune system, viral infections, fatigue, and digestive health.
Study 1
Study type:
Randomised, double-blind, placebo-controlled study
Purpose:
To evaluate the ability of turkey tail polysaccharide peptide to relieve symptoms of advanced lung cancer.
Dose:
3.06 g/day of turkey tail polysaccharide peptide (3 x 3 x 340 mg capsules; three capsules thrice a day)
Participants:
68 patients with non-small cell lung cancer, aged 34-82 years; 58 patients were evaluated
Duration:
4 weeks
Results:
The researchers observed significant improvements in various health indicators among the patients receiving turkey tail mushroom, while no such improvements were observed in the control group. These improvements included increased white blood cell counts, elevated levels of immune system proteins (serum IgG and IgM), and a reduction in body fat percentage. Increased white blood cells, as well as elevated levels of immune system proteins like serum IgG and IgM, are beneficial because they show that the immune system is actively responding to potential threats and is working to protect the body from infections and diseases.
Year:
2003
Link:
Study 2
Study type:
Case report
Purpose:
To investigate how turkey tail mushroom supplements and chemotherapy affect and change the functioning of the immune system of patients with breast cancer.
Dose:
4 g/day of turkey tail mushroom and a 17-species mushroom formula containing polysaccharides (beta-glucans, arabinoxylane, glucose, xylose, galactose, cordycepic acid, glycoproteins, ergosterols, triterpenoids, and other myconutrients).
Additional treatments:
Maintenance therapy with Herceptin (a medication used in the treatment of certain types of breast cancer) every 3 weeks
Participant:
An 83-year-old woman diagnosed in June 2009 with advanced, metastatic inflammatory breast cancer.
Results:
The case report highlighted that the patient, who took a daily dose of turkey tail mushroom alongside a combined mushroom formula and Herceptin treatment for three and a half years, maintained an active and vibrant lifestyle and remained free of the disease.
Year:
2012
Link:
Study 3
Study type:
Rodent study (mice with immunodeficiency)
Purpose:
To investigate the anti-tumor effects of a substance extracted from turkey tail mushroom called protein-bound polysaccharide K. Protein-bound polysaccharide K has been studied for its potential health benefits, including improvements in the functioning of the immune system.
Dose:
20 mg/kg three times daily of protein-bound polysaccharide K from turkey tail or control (saline/saltwater solution)
Duration:
17 days
Results:
The protein-bound polysaccharide K from the turkey tail led to a significant reduction in tumour growth compared to the control group on day 17 after tumour implantation.
The researchers also found that protein-bound polysaccharide K from the turkey tail not only reached the tumour tissue, but also the spleen and liver.
Year:
2011
Link:
Study 1
Study type:
Preliminary clinical trial
Purpose:
To investigate the effects of turkey tail in combination with reishi mushroom on oral human papillomavirus (HPV). Oral HPV is a sexually transmitted disease that mostly spreads through oral sex or mouth-to-mouth contact.
Dose:
200 mg/day of turkey tail and reishi mushrooms (2 x 100 mg capsules) or 400 mg/day of chicken of the woods mushroom (2 x 200 mg capsules)
Participants:
61 oral HPV positive patients
Duration:
2 months
Results:
The researchers observed that in the group receiving turkey tail and reishi mushroom treatment, 87.8% of the cases showed clearance of oral HPV after 2 months of administration. In contrast, in the control group (treated with chicken of the woods mushroom), only 5% of the cases showed clearance of oral HPV. No adverse events were reported during the study. The results suggest that the combination of turkey tail and reishi mushroom may be an effective treatment for eliminating oral HPV infections.
Year:
2014
Link:
Study 1
Study type:
Randomised clinical trial
Purpose:
To compare the effects of turkey tail mushroom polysaccharopeptide to a commonly used antibiotic medication (amoxicillin) on the human gut microbiome. The human gut microbiome is a complex collection of microorganisms in the digestive tract that influence digestion, metabolism, immune function, and mental health, contributing to overall well-being.
Dose:
3600 mg/day of turkey tail mushroom polysaccharopeptide (3 x 1200 mg capsules) or 750 mg/day of amoxicillin (3 x 250 mg) or placebo
Participants:
22 healthy volunteers with an average age of 31 years
Duration:
8 weeks
Results:
In healthy individuals, the gut microbiome, which refers to the vast community of microorganisms living in the digestive tract, is incredibly diverse, meaning there is a vast array of different microorganisms. The diversity of the gut microbiome is essential for its proper functioning and its ability to support overall health.
In the study, antibiotic treatment caused substantial, unfavourable microbiome changes, most notably an increase in Escherichia (E. coli)/Shigella. These changes persisted to the end of the study, 42 days after antibiotic therapy ended. Recovery from this disruption to the microbiome can take several weeks.
Turkey tail treatment, on the other hand, was found to have the ability to modulate or change the composition of the microbiome in a way that promotes a healthier balance of microorganisms. By acting as a prebiotic (a type of dietary fibre that serves as food for beneficial gut bacteria), turkey tail polysaccharopeptide may positively influence the diversity and overall composition of the microbiome in the human gut.
Year:
2014
Link:
Study 1
Study type:
Animal study (mice)
Purpose:
To investigate the effect of turkey tail mycelium extract on exercise performance and physical fatigue in mice.
Dose:
615, 1230 or 3075 mg/kg/day of turkey tail mycelium extract or control (distilled water)
Duration:
4 weeks
Results:
Turkey tail mycelium had positive effects on exercise performance and fatigue in mice. There was a significant increase in forelimb grip strength in mice treated with turkey tail, compared to the control group. After engaging in swimming, mice treated with turkey tail exhibited lower blood levels of molecules called creatine kinase, serum lactate, and ammonia, in comparison to the control group. Lower levels of these molecules can indicate improved exercise performance, reduced muscle fatigue, and enhanced recovery. Higher levels of these molecules are often associated with fatigue and muscle fatigue. Further, turkey tail supplementation favourably lowered blood sugar levels and did not result in any kidney damage.
Overall, the findings suggest that turkey tail mycelium extract may have potential benefits in enhancing physical performance, reducing fatigue, and maintaining metabolic health.
Year:
2017
Link:
Reishi Scientific Studies
The effects of Reishi have been examined in various studies. We have summarised the most interesting ones related to immune system, fatigue, blood pressure, heart health, liver disease, urinary tract infections, and viral infections.
Study 1
Study type:
Systematic review
Purpose:
To explore the anti-neuroinflammatory activities of bioactive compounds from medicinal mushrooms, including reishi in various laboratory studies and animal studies.
Results:
Various studies have shown that reishi mushroom extracts have anti-neuroinflammatory properties, meaning that they have properties that counteract inflammation in the brain and nervous system. Neuroinflammation plays a major role in Alzheimer's Disease, as well as Parkinson's Disease, brain ischemia, epilepsy and depression.
A study revealed that reishi extracts reduced levels of IL-8 and TNF-α, which are small proteins called cytokines that play a role in the immune response and inflammation within the hippocampus. The hippocampus is a region of the brain that is important for learning and memory, and is often affected in neurodegenerative diseases such as Alzheimer’s. In the context of neuroinflammation, IL-8 and TNF-α can contribute to damage and degeneration of brain cells. Reduced levels of these cytokines suggests that the reishi extract has anti-inflammatory effects in the brain.
Another study found that reishi spore powder and lion’s mane extract had protective effects on neurons and the broader nervous system, (“neuroprotective” effects) and reduced neuronal apoptosis (reduced brain cell death) in animal models of epilepsy.
Year:
2020
Link:
Study 2
Study type:
Single case study
Purpose:
To evaluate the effects of self-medication with reishi on the symptoms of Parkinson's disease, a neurodegenerative disorder that primarily affects movement control. Parkinson's disease can also cause non-motor symptoms, including cognitive changes, depression, anxiety, sleep disturbances, and gastrointestinal issues.
Method of evaluation:
Symptoms of Parkinson’s disease were assessed using self-administered questionnaires which measure motor and behavioural aspects of the disease, quality-of-life, self-compassion, positive emotions, and multiple aspects of emotional dysregulation such as nonacceptance of emotional responses, difficulties engaging in goal-directed behaviour, impulse control difficulties, lack of emotional awareness, limited access to emotion regulation strategies, and lack of emotional clarity.
Dose:
300 mg/day of reishi
Duration:
3 months
Results:
The patient reported an increase in mindfulness from “moderate” at the start of the study to “high” after 3 months of self-medication with reishi. Mindfulness is a mental state characterised by heightened awareness of the present moment, an open and non-judgmental attitude, and a focus on one's thoughts, sensations, and surroundings. In the context of Parkinson's disease, high mindfulness could imply that these patients are better able to cultivate and sustain these qualities, which may have positive effects on their overall well-being and symptom management.
The patient also reported moderate self-compassion scores throughout the study, with high scores for self-kindness, which can be an important aspect of coping with Parkinson's disease.
Moreover, the patient's satisfaction with the treatment was rated with scores of 60% (Effectiveness), 100% (Convenience) and 85% (Overall Satisfaction). The patient did not report any side effects of the treatment. Motor symptoms remained stable, with no extreme changes in quality of life.
These positive changes in the patient's affective behaviour and overall treatment satisfaction may suggest potential benefits of reishi supplementation for cognitive health.
Year:
2021
Link:
Study 3
Study type:
Cellular and animal study
Purpose:
To investigate the effects of reishi triterpenoids (organic compounds found in reishi) on cognitive impairment in an Alzheimer's mice model.
Dose:
Animal study: 0.35 and 1.40 mg/kg of reishi triterpenoids or controls
Cellular study: 30 and 300 μmol/L of reishi triterpenoids or control
Duration:
60 days
Results:
In the animal experiment, the results found that the administration of reishi triterpenoids reduced cognitive impairment and improved spatial learning in mice. Spatial learning refers to the process through which an organism acquires a mental representation of its environment.
In the cellular experiment, significant hippocampal damage was observed in mice with Alzheimer’s disease, however reishi triterpenoids mitigated this damage by inhibiting apoptosis, the process of cell death. Inhibiting the process of cell death can help protect neurons and prevent their premature death, thereby preserving brain function and memory. Additionally, reishi triterpenoids restored the levels of antioxidative proteins and deactivated the ROCK signalling pathway, which is involved in various cellular processes, including neuroinflammation, often observed in Alzheimer's disease. By deactivating this pathway, it is possible to reduce inflammation and cellular stress, contributing to the overall reduction of neurodegenerative processes and cognitive decline.
Overall, the results suggest that reishi triterpenoids have the potential to improve cognitive impairment, reduce neuronal damage, and inhibit the process of cell death in the brain and cells of mice with Alzheimer’s disease.
Year:
2020
Link:
Study 4
Study type:
Rodent study
Purpose:
To investigate the therapeutic effects of reishi on cognitive function in a mouse model of Alzheimer's disease.
Method of evaluation:
Spatial learning and memory were evaluated using the Morris water maze, where mice had to use environmental cues to locate a hidden platform. The time taken to find the platform, known as escape latency, was measured. Longer escape latencies indicate learning difficulties or memory impairment, while shorter escape latencies suggest better learning and memory abilities.
Dose:
30 mg/kg/day of reishi polysaccharides or 200 mg/kg of reishi water extract or control
Duration:
90 days
Results:
Mice treated with reishi (both polysaccharides and water extract) exhibited improved spatial learning memory, a type of memory essential for navigation, orientation, and understanding the layout of spaces. This was established by the less time they spent locating the hidden platform in the Morris maze test. The study also found that reishi polysaccharides reduced cognitive deficits and enhanced neurogenesis (the process of generating new neurons, which is involved in functions such as learning, memory, and the brain's ability to adapt and think flexibly) in mice with Alzheimer’s disease.
Reishi oral supplementation also improved cognitive deficits in the mice and promoted the proliferation (number) of “neural progenitor cells”, which are cells that play a pivotal role in brain development, repair, and regeneration, leading to the development of new neurons.
Year:
2017
Link:
Study 5
Study type:
Rodent study
Purpose:
To investigate the effects of reishi on rats with Alzheimer’s disease induced by streptozotocin, a chemical compound, injected directly into the brain.
Dose:
2.0, 4.0, 8.0 g/kg body weight of reishi spore (pre-administered before streptozotocin injury) or control
Duration:
3 weeks
Results:
Rats injected with streptozotocin but not treated with reishi, displayed elevated oxidative stress (characterised by an increase in free radicals that can cause damage to cells, proteins, and DNA) along with impaired functioning of mitochondria, the energy-producing “powerhouses” within cells. In the context of Alzheimer's disease, mitochondrial dysfunction is linked to neuronal and cell damage, which are key factors in the progression of the disease. Furthermore, these rats exhibited deficits in spatial learning and memory, as well as severe damage to hippocampal neurons.
On the other hand, rats treated with reishi at a dosage of 8.0 g/kg exhibited significant reversal of these abnormalities, suggesting that reishi may have protective effects on the hippocampus against oxidative damage and energy metabolism disruption caused by streptozotocin.
Overall, the findings suggest that reishi could have potential therapeutic effects in the treatment of neurodegenerative disorders such as Alzheimer's disease.
Year:
2012
Link:
Study 6
Study type:
Rodent study
Purpose:
To investigate the protective effects of reishi spore extract against learning and memory impairments in rats with Alzheimer's disease induced by streptozotocin, a chemical compound which contributes to neurodegenerative processes (gradual and progressive damage of nerve cells in the brain).
Method of evaluation:
Learning and memory abilities in mice were evaluated using the Morris water maze, a task in which mice had to use environmental cues to locate a hidden platform. The time taken to find the platform, known as escape latency, was measured. Longer escape latencies indicate learning difficulties or memory impairment, while shorter escape latencies suggest better learning and memory abilities.
Dose:
360 and 720 mg/kg of reishi spore extract or control
Duration:
21 days (14 days of reishi treatment)
Results:
The study found that treatment with reishi spore extract significantly improved memory in rats with Alzheimer's disease. Reishi spore extract reversed the increases in proteins associated with Alzheimer's disease (Amyloid β and Tau proteins) and restored levels of neurotrophic factors (often called 'growth factors' for neurons) in the hippocampus, a region of the brain known for its crucial role in memory, learning, and emotional regulation.
Furthermore, based on the Morris water maze test, rats treated with reishi extract demonstrated shorter latencies in reaching the hidden platform compared to the control group. Shorter escape latencies indicate improved learning and memory abilities.
Overall, the study highlights the potential of reishi spore extract to enhance memory, improve protein imbalances associated with Alzheimer's disease, and restore neurotrophic signalling in the hippocampus (the communication system in the brain's memory centre), suggesting a potential benefit for addressing cognitive deficits.
Year:
2021
Link:
Study 7
Study type:
Rodent study
Purpose:
To evaluate the effect of reishi on memory and learning tasks in male mice with memory impairment.
Dose:
150 and 300 mg/kg of reishi ethanol extract or 0.1 mg/kg of physostigmine (a commercial drug used to treat conditions related to disruptions in the normal balance of neurotransmitters in the brain) or controls
Duration:
11 days
Results:
Results from the learning and memory tests found that mice treated with reishi extract demonstrated improved learning and memory compared to control mice. The effects were more significant at the higher dose of 300 mg/kg compared to 150 mg/kg.
In addition, the activity of acetylcholinesterase (an enzyme that breaks down the neurotransmitter acetylcholine) in the brain was significantly increased in the control group, but was significantly reduced when reishi extract or physostigmine were administered. Certain medications used to address conditions like Alzheimer's disease function by inhibiting acetylcholinesterase activity, thereby increasing brain acetylcholine levels and potentially enhancing memory and cognitive functions in affected individuals.
Overall, the study revealed that reishi may have nootropic effects, improving thinking, learning, and memory.
Year:
2019
Link:
Study 8
Study type:
Rodent study
Purpose:
To investigate the effects of long-term supplementation with reishi triterpenoids (an organic compound found in reishi) on age-associated brain decline in two Alzheimer's mice models.
Dose:
25 or 100 mg/kg/day of reishi triterpenoids or ganodenic acid A (a specific compound, a “triterpenoid”, from reishi) or controls.
Duration:
10 months
Results:
The study found that the long-term treatment with reishi triterpenoids improved age-associated brain decline in normal ageing mice. The researchers also noticed that reishi treatment increased the activity (expression) of proteins that play a role in cleaning up damaged components within cells (a process called autophagy). This helps to clear accumulations and clumpings of misfolded proteins in the brain, a prominent feature of neurodegenerative diseases like Alzheimer’s, potentially slowing the progression of these diseases.
Ganoderic acid A, a reishi triterpenoid, was found to be potentially effective in improving brain function in the mice model of Alzheimer's Disease, which suggests promising avenues for improving cognitive health.
Year:
2021
Link:
Study 9
Study type:
Rodent study
Purpose:
To investigate the effects of reishi extract on the behavioural responses of mice with behavioural disorders induced by alcohol.
Method of evaluation:
In this study, the behavioural responses and memory abilities of mice were assessed using four behavioural tests that measured the mice's motor performance, anxiety- and depression-like symptoms.
Dose:
300 mg/kg/day of reishi extract or control (distilled water)
Duration:
3 days
Results:
The study discovered that reishi extract helped alleviate behavioural disorders induced by alcohol in mice. This included reducing anxiety and depression-like symptoms, enhancing short-term memory, and boosting motor performance. Resihi aqueous extract also improved the animals' REDOX balance, indicating its antioxidant properties. REDOX balance involves maintaining equilibrium between harmful molecules that can damage cells (reactive oxygen and nitrogen species) and protective molecules that prevent this damage (antioxidants). Excessive or chronic alcohol intake often disrupts the redox balance in the body. Overall, the findings highlight the potential of reishi extract in reducing alcohol-induced behavioural and oxidative disturbances.
Year:
2020
Link:
Study 10
Study type:
Cellular study
Purpose:
To investigate the neuroprotective effects of reishi polysaccharides against oxidative stress-induced brain cell death (neuronal apoptosis). Oxidative stress is a state of imbalance between the production of reactive oxygen species (harmful molecules) and the body's ability to counteract their harmful effects. When cells are exposed to oxidative stress, they undergo cell death (apoptosis), which can lead to tissue damage and disease. Many studies have found that Alzheimer's disease is associated with brain cell death (neuronal apoptosis), contributing to the cognitive and memory problems seen in Alzheimer's patients.
Dose:
0.5 g, 2 g, or 5 g of reishi per 100 mL solution
Results:
Reishi polysaccharides were found to inhibit cell death (apoptosis) in cerebellar granule cells (neurons located in a part of the brain called the cerebellum) that were exposed to hydrogen peroxide, a common trigger of oxidative stress. Treatment with reishi polysaccharides reduced the activity (the expression) of proteins linked to cell death. Reishi polysaccharides also promoted the production of the Bcl-2 protein, which prevents cell death (a so-called “an anti-apoptotic” protein). The findings provide new insights into the neuroprotective mechanisms of reishi and support its potential use in treating neurodegenerative diseases involving oxidative stress.
Year:
2017
Link:
Study 1
Study type:
Rodent study
Purpose:
To investigate the effects of reishi extract on the behavioural responses of mice with behavioural disorders induced by alcohol.
Method of evaluation:
In this study, the behavioural responses and memory abilities of mice were assessed using four behavioural tests that measured the mice's motor performance, anxiety- and depression-like symptoms.
Dose:
300 mg/kg/day of reishi extract or control (distilled water)
Duration:
3 days
Results:
The study discovered that reishi extract helped alleviate behavioural disorders induced by alcohol in mice. This included reducing anxiety and depression-like symptoms, enhancing short-term memory, and boosting motor performance. Resihi aqueous extract also improved the animals' REDOX balance, indicating its antioxidant properties. REDOX balance involves maintaining equilibrium between harmful molecules that can damage cells (reactive oxygen and nitrogen species) and protective molecules that prevent this damage (antioxidants). Excessive or chronic alcohol intake often disrupts the redox balance in the body. Overall, the findings highlight the potential of reishi extract in reducing alcohol-induced behavioural and oxidative disturbances.
Year:
2020
Link:
Study 2
Study type:
Rodent study
Purpose:
To investigate the antidepressant effect of reishi in rats.
Method of evaluation:
Anxiety and antidepressant-like responses in rats were evaluated through various behavioural tests, which included the open-field test, forced swimming test, elevated plus maze, contextual fear-conditioning, and head-twitch test. The open-field test quantified physical movement; the forced swimming test evaluated reactions in a water container indicating mood and motivation; the elevated plus maze determined anxiety levels and emotional responses; contextual fear-conditioning examined fear responses in connection to a shock-related environment; and the head-twitch test focused on behaviours tied to serotonin receptors These tests collectively provided insights into mood, motivation, fear memory, and neuropsychiatric conditions.
Dose:
0.3 and 1.0 g/kg body weight of reishi or control
Duration:
Single administration (60 min before the tests)
Results:
This study suggests that reishi could potentially act as an antidepressant and alleviate anxiety. The observed reductions in immobility during the forced swimming test and freezing behaviour in the contextual fear-conditioning test after reishi administration suggest an improved mood and a reduction in fear and anxiety. Furthermore, the observed decrease in head twitches during the head-twitch test indicates a calming effect on neurological signals, meaning decreased anxiety and stress. While no statistically significant response was noted in locomotion (physical behaviour) or anxiety-like behaviour during evaluations in the open-field or elevated plus-maze tests, the overall findings show reishi may have promising effects on emotional well-being.
Year:
2013
Link:
Study 1
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To investigate the effects of β-glucan derived from reishi mushrooms in healthy adults. β-glucan is a complex carbohydrate that possesses properties that can assist in regulating and boosting the immune system.
Dose:
200 mg/day of reishi β-glucan or placebo
Participants:
135 healthy adults, aged 18-55 years
Duration:
84 days
Results:
Researchers found an association between reishi β-glucan treatment and significant increases in various antibodies and immune cells. These results indicate a strengthened immune defence, helping to protect the body against infections and diseases. Additionally, the researchers observed a notable 83.1% increase in NK cell-mediated cytotoxicity in the intervention group compared to the placebo. An increase in NK cell-mediated cytotoxicity means that the natural killer (NK) cells in the immune system are more effective at identifying and destroying abnormal or infected cells. These findings suggest that reishi β-glucan may have a positive impact on the immune system.
Year:
2023
Link:
Study 2
Study type:
Randomised, double-blind, controlled, clinical trial
Purpose:
To assess the effects of reishi mushroom in patients with postoperative lung and breast cancer.
Dose:
4000 mg/day of reishi mushroom (2 x 2000 mg spore powder) or placebo
Participants:
120 breast and lung cancer patients undergoing chemotherapy, aged 37-92 years.
Duration:
6 weeks
Results:
Researchers observed significant increases in immune response molecules - IL-2 (Interleukin-2) and IL-12 (Interleukin-12) - after reishi supplementation and chemotherapy. An increase in IL-2 and IL-12 levels can enhance the immune response by promoting the activation, proliferation, and coordination of various immune cells. This can be beneficial in fighting infections, supporting cancer immunotherapy, and maintaining immune system balance. The researchers also observed a notable decrease in immune-suppressive molecules.
Overall, the results indicate that reishi mushroom combined with chemotherapy may positively impact the immune system by boosting beneficial immune cells and reducing those that hinder immune function.
With the exception of slight discomfort (such as a dry mouth), no serious adverse effects were observed during the study.
Year:
2020
Link:
Study 3
Study type:
Single-arm, clinical trial (uncontrolled)
Purpose:
To investigate the effects of the polysaccharides extracted from reishi mushroom on the immune function in patients with advanced-stage cancer.
Dose:
1800 mg/day of reishi mushroom extract (3 x 600 mg capsules with 25% polysaccharides)
Participants:
30 patients with advanced-stage cancer, aged 31-77 years
Duration:
12 weeks
Results:
Researchers observed significant increases in the levels of cytokines (IL-2, IL-6, IFN-g) after reishi supplementation. Cytokines are small proteins that play essential roles in the body’s immune system. Higher levels of these cytokines strengthen the immune system, enhancing the body's ability to fight infections, regulate inflammation, and support overall immune system functioning.
The researchers also observed a notable rise in a type of immune cell called CD56+ cells, which are known to play a role in fighting infections and cancer. Natural killer (NK) cells also became more active, which are specialised in killing harmful cells.
These findings suggest that reishi mushroom treatment may have beneficial effects on the immune system and could potentially enhance the body's ability to defend against diseases.
Year:
2003
Link:
Study 4
Study type:
Cellular study (in-vitro)
Purpose:
To assess the protective effects of reishi polysaccharides against lymphocyte suppression caused by plasma from lung cancer patients. Lymphocyte suppression refers to a condition where the body's immune cells, known as lymphocytes, are decreased in their numbers or activity, which can weaken the immune system's ability to fight off infections and diseases.
Dose:
0.2, 0.8, 3.2, and 12.8 micrograms/mL
Participants:
Blood was collected from 12 lung cancer patients with an average age of 57 years.
Results:
Blood plasma (the liquid part of blood) from patients with lung cancer was found to have a suppressing effect on lymphocyte proliferation (lymphocyte multiplication), which weakens the immune system. Additionally, the plasma showed reduced activity of certain proteins that are crucial for the immune system to fight cancer cells effectively.
However, these effects were partially or fully reversed after the addition of reishi polysaccharides. These findings suggest that reishi polysaccharides may help counteract the immune suppressive effects in lung cancer patients.
Year:
2014
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To assess the effects of reishi polysaccharide extract on patients with a condition called neurasthenia, which is characterised by chronic fatigue, mental and physical exhaustion, and various symptoms associated with prolonged stress and strain.
Method of evaluation:
The severity and improvements in the health condition under study (neurasthenia) were assessed using the Clinical Global Impression scale, which measures symptom severity, treatment response and the efficacy of treatments.
Well-being and fatigue were assessed using a self-reported rating scale which measures subjective sense of well-being and the sense of fatigue.
Dose:
1800 mg/day of reishi mushroom extract (3 x 600 mg capsules, with 25% (wt/wt) crude polysaccharides
Participants:
123 patients with neurasthenia, aged 18-65 years
Duration:
8 weeks
Results:
The study found an association between 8 weeks of reishi mushroom extract supplementation and a lower sense of fatigue (28.3% reduction) and lower scores in the Clinical Global Impression severity score (15.5% reduction) from baseline. Lower scores in the Clinical Global Impression indicate that the participants' overall condition or severity of fatigue has improved. In contrast, the reductions in the placebo group were less significant: 4.9% and 20.1%, respectively.
The researchers also observed that the sense of well-being score at day 56 increased by 38.7% in the reishi-treated group compared to the 29.7% increase in the placebo group.
These findings suggest that reishi treatment may be effective in improving the sense of well-being and reducing fatigue in neurasthenic patients.
Year:
2005
Link:
Study 2
Study type:
Randomised controlled pilot trial
Purpose:
To evaluate the effects of reishi spore powder on fatigue and overall quality of life in patients with breast cancer undergoing endocrine therapy. Reishi spore powder is a concentrated form of bioactive compounds derived from the spores of Reishi mushrooms.
Method of evaluation:
Fatigue and quality of life were assessed using self-reported questionnaires, which assess fatigue, anxiety, depression and quality of life in patients undergoing cancer therapy. Blood samples were also collected during, before, and after treatment to determine the concentrations of fatigue markers.
Dose:
3000 mg of reishi powder (3 x 1000 mg spore powder) or placebo
Participants:
48 breast cancer patients with cancer-related fatigue with an average age of 52 years
Duration:
4 weeks
Results:
The study found an association between reishi powder supplementation and significant improvements in physical well-being and fatigue. Patients treated with reishi reported reduced levels of anxiety and depression, along with an enhanced quality of life, including improvements in sleep and appetite. The researchers also observed that the immune markers associated with cancer-related fatigue were notably lower, and no serious adverse effects were observed throughout the study. This pilot study suggests that reishi powder may have positive effects on cancer-related fatigue and quality of life in breast cancer patients undergoing endocrine therapy, without significant adverse effects.
Year:
2012
Link:
Study 3
Study type:
Randomised, double-blinded, clinical trial (uncontrolled)
Purpose:
To compare the effects between reishi mushroom and carob tree on physical fitness in patients suffering from fibromyalgia, a chronic disorder characterised by widespread musculoskeletal pain, fatigue, and tenderness in specific areas of the body.
Dose:
6 g/day of reishi mushroom or 6 g/day of carob tree extract
Participants:
48 women with fibromyalgia with an average age of 55 years
Duration:
6 weeks
Results:
The study found that treatment of 6g/day of reishi mushroom for 6 weeks is associated with improved physical fitness in women suffering from fibromyalgia. Specifically, the researchers observed improvements in aerobic endurance (stamina), walking speed, and lower limb flexibility after reishi mushroom treatment. On the other hand, no association was observed between carob tree extract and improvements on physical fitness.
Year:
2015
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled crossover trial
Purpose:
To assess the antioxidative and liver-protective effects of reishi in volunteers with mild liver dysfunction.
Dose:
225 mg/day of reishi enriched with triterpenoids and polysaccharide peptides (1 x 225 mg capsules, after lunch or dinner) or placebo. Triterpenoids and polysaccharide peptides are natural compounds found in Reishi mushrooms that have potential health benefits, with triterpenoids contributing to its anti-inflammatory and antioxidant properties, while polysaccharide peptides may support immune function.
Participants:
39 volunteers with mild liver dysfunction, aged 40-54 years
Duration:
6 months
Results:
The study found a significant association between reishi supplementation and a reduction in the levels of various oxidative stress markers linked to premature ageing and cellular damage. Oxidative stress markers are indicators used to assess the level of cellular damage caused by harmful molecules called free radicals.
Researchers also observed an increase in the levels of various anti-oxidative enzymes that help suppress the activity of free radicals. Antioxidants help neutralise these free radicals to safeguard our health.
After reishi supplementation, there was an improvement in the structure and form of the liver, as well as a normalisation of liver damage.
Overall, these findings suggest that reishi may possess antioxidative, anti-aging, and liver-protective effects by counteracting the excessive production of free radicals and safeguarding cells against damage.
Year:
2017
Link:
Study 1
Study type:
Randomised, double-blind, cross-over clinical trial
Purpose:
To investigate the beneficial effects of reishi on a wide range of cardiovascular and metabolic parameters in patients with elevated blood pressure and/or cholesterol.
Dose:
1.44 g/day of reishi extract (4 x 360 mg capsules, two capsules twice daily) or placebo
Participants:
25 patients with high blood pressure and/or abnormal levels of lipids (fats) in the bloodstream, particularly elevated levels of cholesterol and triglycerides.
Duration:
12 weeks
Results:
The researchers observed an 8% decrease in triglycerides and a 28% increase in HDL-cholesterol (“good” cholesterol) following reishi supplementation during the first treatment period, whereas these changes were not observed in the placebo group. High triglycerides are considered a risk factor for various health conditions, while low HDL-cholesterol may indicate an increased risk of cardiovascular problems. Therefore, the decrease in triglycerides and increase in HDL-cholesterol indicate a positive impact on lipid profile and cardiovascular health.
Both the reishi and placebo treatments were well tolerated, and no adverse effects in the laboratory safety parameters were detected.
Year:
2012
Link:
Study 2
Study type:
Single-blinded, quasi experimental trial (uncontrolled)
Purpose:
To investigate the antioxidant effect of reishi polysaccharide peptide against atherosclerosis, the thickening or hardening of the arteries, in patients with angina. Angina is a symptom of coronary artery disease, often described as squeezing, pressure, heaviness, tightness or pain in the chest.
Dose:
750 mg/day of reishi polysaccharide peptide (3 x 250 mg freeze dried preparations)
Participants:
37 high risk and 34 stable angina patients (stable angina is a form of chest pain that occurs during physical or emotional stress, while high-risk angina is more severe, unpredictable, and associated with a higher risk of complications)
Duration:
90 days
Results:
The study found an association between reishi polysaccharide supplementation and a significant increase in levels of superoxide dismutase (an enzyme that plays a crucial role in the antioxidant defence system of cells) in patients with stable angina patients, but not in high-risk angina patients. Additionally, the researchers observed a significant decrease in malondialdehyde, a marker of oxidative stress that can cause damage to the cells. Higher superoxide dismutase activity and lower malondialdehyde levels suggest that the body's antioxidant defences are more effectively neutralising harmful substances (free radicals) and preventing damage.
Furthermore, the researchers observed a significant reduction in circulating endothelial cells counts in both patient groups. Circulating endothelial cells are a type of cell that originates from the inner lining (endothelium) of blood vessels. The reduction in circulating endothelial cells counts suggests a potential improvement in the health and function of blood vessels, which is crucial for maintaining cardiovascular well-being.
Overall, these findings suggest that reishi polysaccharide supplementation may have therapeutic potential in managing cardiovascular conditions.
Year:
2018
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To evaluate the effects of reishi mushroom extract in men with mild to-moderate lower urinary tract symptoms .
Method of Evaluation:
Urinary symptoms were assessed using the International Prostate Symptom Score (IPSS), a self-administered questionnaire which measures the severity of urinary symptoms.
Quality of life was assessed using one quality-of-life question for which the answers ranged from ‘delighted’ (0) to ‘terrible’ (6).
The peak urinary flow rate (the maximum speed at which urine flows out during urination) was assessed using a uroflowmeter. This is a measurement that helps evaluate how well the urinary system is functioning
Prostate volume (the size of the prostate) and residual urine volume were measured using ultrasonography, which assesses the size of the prostate gland and measures the amount of urine remaining in the bladder after urination.
Dose:
6 mg/day of reishi mushroom extract (2 x 3 mg of reishi mushroom extract in a 2 g tablet) or placebo
Participants:
88 men with an average age of 64 years
Duration:
12 weeks of treatment with follow up on the 16th week
Results:
The study found an association between 12 weeks of reishi mushroom supplementation and a significant 2.1-point decrease in the International Prostate Symptom Score, compared to a 0.77 decrease in the placebo group. A lower score indicates a decrease in urinary symptoms such as frequency, urgency, weak urine flow, and nighttime urination, reflecting an improvement in the overall urinary function and quality of life.
Additionally, quality of life scores also improved in both groups during the treatment, but no significant difference was found between the groups. No changes were observed in peak urinary flow, mean urinary flow, residual urine, prostate volume, serum prostate-specific antigen, or testosterone levels. Overall, the treatment was well tolerated, with no severe adverse effects reported.
Year:
2008
Link:
Study 2
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To evaluate the effects of reishi mushroom extract in men with lower urinary tract symptoms.
Method of evaluation:
Urinary symptoms were assessed using the International Prostate Symptom Score (IPSS), a self-administered questionnaire which measures the severity of urinary symptoms.
Quality of life was assessed using one quality-of-life question for which the answers ranged from ‘delighted’ (0) to ‘terrible’ (6).
The peak urinary flow rate (the maximum speed at which urine flows out during urination) was assessed using a uroflowmeter. This is a measurement that helps evaluate how well the urinary system is functioning
Prostate volume (the size of the prostate) and residual urine volume were measured using ultrasonography, which assesses the size of the prostate gland and measures the amount of urine remaining in the bladder after urination.
Dose:
0.6, 6, and 60 mg/day of reishi mushroom extract or placebo
Participants:
50 men with lower urinary symptoms, aged 50-70 years
Duration:
8 weeks of treatment with follow up on the 10th week
Results:
The study found an association between reishi supplementation (6mg and 60mg) and significant reductions in the total International Prostate Symptom Score (and therefore reductions in the severity of urinary symptoms), as well as improved quality of life at weeks 4 and 8. A lower score in the questionnaires indicates improved urinary function and quality of life due to reduced urinary symptoms such as frequency, urgency, weak urine flow, and nighttime urination. At week 10, the researchers observed that scores from both questionnaires were the lowest after the 6 mg dose. However, there were no significant changes in prostate-specific antigen levels and prostate volume, and no significant improvements were observed in peak urinary flow rate and post-void residual urine volume among the four groups.
Overall, treatment with 6mg and 60mg doses were both associated with improvements in urinary tract symptoms and quality of life.
Year:
2008
Link:
Study 1
Study type:
Preliminary clinical trial
Purpose:
To investigate the effects of turkey tail in combination with reishi mushroom on oral human papillomavirus (HPV), the most common sexually transmitted disease, mostly spread through oral sex or mouth-to-mouth contact.
Dose:
200 mg/day of turkey tail and reishi (2 x 100 mg capsules) or 400 mg/day of chicken of the woods mushroom (2 x 200 mg capsules)
Participants:
61 oral HPV positive patients
Duration:
2 months
Results:
The researchers observed that in the group receiving turkey tail and reishi mushroom treatment, 87.8% of the cases showed clearance of oral HPV after 2 months of administration. In contrast, in the control group (treated with chicken of the woods mushroom), only 5% of the cases showed clearance of oral HPV. No adverse events were reported during the study. The results suggest that the combination of turkey tail and reishi mushroom may be an effective treatment for eliminating oral HPV infections.
Year:
2014
Link:
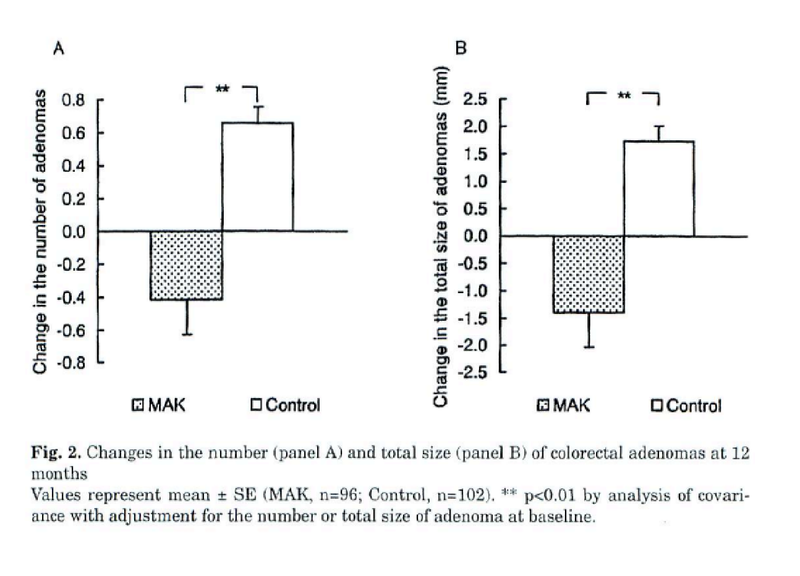
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To evaluate the effects of reishi mushroom mycelia in patients with colorectal adenomas, which are tumours that develop in the lining of the colon or rectum.
Dose:
1.5 g/day of reishi mushroom mycelia (6 x 0.25 g capsules, three capsules twice daily)
Participants:
198 patients with colorectal adenomas with an average age of 64 years
Duration:
12 months
Results:
The study found an association between reishi supplementation and a significant decrease in the number and total size of adenomas, which are abnormal growths that can occur in various tissues, including the colon. A lower number of adenomas is considered a positive outcome as it indicates a reduced risk of cancer development or progression. Participants taking reishi experienced a 52% reduction in the number of adenomas, while the control group only experienced a 2% decrease. These findings suggest that reishi mushroom supplements may have potential benefits in preventing or managing adenomas and reducing the risk of cancer.
Year:
2010
Link:
PRODUCTS CONTAINING REISHI
Reishi Capsules

Chaga Scientific Studies
Several rodent and cellular studies have investigated the effects of chaga mushroom. We have compiled the most interesting results related to fatigue, perfromance enhancement, diabetes, tumours, testosterone, antioxidants, inflammation, allergies, liver diease, heart health, cognitive health, and weight management.
Study 1
Study type:
Rodent study
Purpose:
To evaluate the effects of chaga mushroom in mice with cognitive dysfunction. Cognitive dysfunction was induced by scopolamine, a medication that blocks neurotransmitters in the brain which disrupts normal memory processes, leading to temporary or transient amnesia.
Method of evaluation:
Memory in mice was assessed using the Passive Avoidance Task, where the mice are placed in a two-compartment apparatus and learn to avoid the unfavourable compartment after experiencing an unpleasant event. Memory performance was measured by “step-through latency”, which reflects the time taken to move between compartments. A shorter step-through latency suggests reduced memory retention or learning ability, while a longer time indicates better memory function.
Spatial learning and memory were evaluated using the Morris water maze, where mice had to use environmental cues to locate a hidden platform. The time taken to find the platform, known as escape latency, was measured. Longer escape latencies indicate learning difficulties or memory impairment, while shorter escape latencies suggest better learning and memory abilities.
Dose:
50 and 100 mg/kg/day doses orally of chaga extract
Duration:
7 days
Results:
The study revealed that mice treated with scopolamine, a medication that blocks neurotransmitters in the brain which disrupts normal memory processes, had lower step-through latencies and escape latencies, indicating impaired memory. However, treatment with chaga mushroom partially restored the lower step-through latency, showing approximately 57% and 72% recovery at the doses of 50 and 100 mg/kg respectively. Escape latencies and swimming time also increased by 65% and 67%, indicating better memory function and learning abilities.
Additionally, the results of the study demonstrated that chaga mushroom extract reduced brain stress, restored essential brain-protective substances, and decreased acetylcholinesterase activity, an enzyme associated with memory problems.
Year:
2011
Link:
Study 2
Study type:
Rodent study
Purpose:
To assess the neuroprotective effects of chaga mushroom against Alzheimer’s disease-like behaviours in APP/PS1 (amyloid precursor protein/presenilin 1) mice. APP/PS1 mice are genetically engineered mice that exhibit several key characteristics of Alzheimer's disease.
Method of evaluation:
In the study, the learning and memory abilities of mice were assessed using three behavioural tests.
Dose:
25 and 50 mg/kg/day of chaga mushroom polysaccharide or control (double distilled water)
Duration:
8 weeks
Results:
The study showed that chaga mushroom polysaccharide treatment had positive effects on mice in various behavioural tests. The treated mice performed better in tests that measured their memory, exploration, and decision-making abilities. The study also discovered that chaga mushroom polysaccharide had a protective effect on cells that were exposed to a harmful substance called L-Glutamate, which can cause damage to cells in diseases that affect the brain.
Overall, the study suggests that chaga mushroom polysaccharide may have beneficial effects on brain function and could potentially be used to develop treatments for diseases that affect memory and cognitive abilities.
Year:
2019
Link:
Study 1
Study type:
Rodent study
Purpose:
To evaluate the potential benefits of chaga mushroom polysaccharides in reducing physical fatigue in mice.
Methods of evaluation:
In this study, researchers used a “forced swim test” where mice were made to swim to see how chaga mushroom polysaccharides affected their ability to handle exercise. The swimming-to-exhaustion test is commonly employed to evaluate the anti-fatigue effects of medications.
Dose:
0, 100, 200, and 300 mg/kg/day of orally-administered chaga mushroom polysaccharides
Duration:
14 days
Results:
The results demonstrated that the chaga polysaccharides-treated groups exhibited significantly longer swimming times until exhaustion compared to the control group. This indicates that chaga polysaccharides effectively enhanced the exercise tolerance of mice (their exercise endurance) and might be a potential anti-fatigue medication .
Furthermore, chaga polysaccharides had no toxic effects on the major organs of the mice, such as the liver.
Year:
2015.
Link:
Study 2
Study type:
Animal study
Purpose:
To investigate the anti-fatigue effects of chaga mushroom polysaccharides in mice.
Methods of evaluation:
In this study, the researchers conducted the forced swim test to investigate the impact of chaga mushroom polysaccharides on exercise endurance in mice. This test is a commonly used model to assess the potential anti-fatigue effects of various medications or substances.
Dose:
50 mg/kg/day of 3 polysaccharide fractions of chaga mushrooms dissolved in distilled water. Chaga mushroom fractions refer to specific components obtained from chaga mushrooms through fractionation or separation techniques. These fractions may have unique properties or compositions that differ from the original mushroom extract.
Duration:
30 days
Results:
The study found that the first polysaccharide fraction of chaga mushroom (fraction 1) exhibited potential anti-fatigue effects. When administered at a dose of 50 mg/kg, fraction 1 increased the duration of climbing and swimming in mice while reducing immobility time. Additionally, fraction 1 led to a decrease in fatigue-related metabolic parameters and significantly reduced the concentrations of serotonin (5-HT) in the mice's brain. Recent research suggests that high release of serotonin is associated with central fatigue, and decreasing serotonin production in the brain may improve endurance exercise performance. On the other hand, the second and third fractions of chaga mushroom did not exhibit any anti-fatigue effect.
Therefore, the study suggests that the first polysaccharide fraction of chaga mushroom may have beneficial effects by reducing fatigue and improving endurance, possibly through its influence on serotonin levels in the brain.
Year:
2020
Link:
Study 1
Study type:
Rodent study
Purpose:
To assess the effects of chaga mushroom supplementation on the body composition of mice in relation to changes in energy levels and exercise performance.
Dose:
824, 1648, and 2472 mg/kg/day of chaga mushroom extract or control
Duration:
6 weeks
Results:
Mice given chaga mushroom had higher levels of free fat mass compared to the other groups. Free fat mass includes all body components except fat, such as water, bone, organs, and muscle mass. Additionally, mice treated with chaga mushroom exhibited a noticeable increase in muscle mass and muscle volume. In terms of exercise performance, the mice that received chaga mushroom treatment showed delayed exhaustion compared to the control group. They also experienced higher rates of glycogen depletion in the liver, as opposed to skeletal muscles. Glycogen depletion in the liver is generally considered more beneficial. It allows the liver to release glucose into the bloodstream to maintain normal blood sugar levels and provide energy to the body's cells, especially when there is an increased demand for energy.
The study also found that chaga mushrooms had positive effects on glucose uptake (sugar uptake) during exercise. Proper glucose uptake is crucial for maintaining proper blood glucose levels and providing energy to cells for their normal functioning.
Chaga mushroom also increased lipid transport in the mice. Lipids are molecules that play important roles in the body. An increase in lipid transport indicates a greater efficiency or capacity for lipid movement, which can have important beneficial effects on various metabolic processes in our body, such as how we break down and use energy.
Year:
2022
Link:
Study 1
Study type:
Rodent study
Purpose:
To investigate the potential effects of chaga mushroom polysaccharide on testicular impairment and disrupted sperm cell development within the testes in male mice infected with Toxoplasma gondii (a parasite). It is important to note that Toxoplasma gondii infects cells involved in sperm production in the testicles, leading to a decrease in sperm quality and potentially affecting male fertility.
Dose:
100, 200, and 400 mg/kg of chaga mushroom polysaccharide or 200 mg/kg Sulfadiazine (a widely used medicine for toxoplasmosis) or control (saltwater solution)
Duration:
7 days
Results:
The study discovered that both chaga mushroom polysaccharide had positive effects on sperm production and testis health. These treatments also raised the levels of important male reproductive hormones involved in male reproduction, such as testosterone, luteinizing hormone, and follicle-stimulating hormone. This increase in hormone levels helped enhance sperm production, hormonal balance, and fertility.
The study also found that chaga mushroom polysaccharide helped reduce oxidative stress (an imbalance between harmful free radicals and the body's ability to neutralize them) and prevented cell death in the testicles. Cell death in the testicles is a significant factor contributing to reproductive damage, which is significantly increased by the parasite Toxoplasma gondii.
The findings suggest that chaga mushroom polysaccharide is effective in enhancing reproductive function in male mice infected with T. gondii.
Year:
2020
Link:
Study 1
Study type:
Rodent study
Purpose:
To explore the antioxidant activities of chaga mushroom polysaccharide and its potential therapeutic effects in mice with chronic pancreatitis (a condition where the pancreas, an organ in your abdomen, becomes inflamed or swollen).
Dose:
100, 200 or 400 mg/kg/day body weight of chaga mushroom
Duration:
4 weeks
Results:
Chaga mushroom polysaccharide had strong antioxidant properties in mice with chronic pancreatitis. It effectively reduced the levels of malondialdehyde in the mice, which is beneficial as high levels of malondialdehyde are associated with increased oxidative stress and cellular damage and dysfunction. Oxidative stress occurs when the production of free radicals exceeds the body's antioxidant defence mechanisms. By reducing these free radicals, chaga mushroom helps protect the cells in the pancreas from harm. Notably, the highest dose of chaga mushroom in the study (400 mg/kg) resulted in the highest increase in levels of superoxide dismutase. Superoxide dismutase is a natural antioxidant that defends cells by converting superoxide radicals, which can cause cellular damage, into less harmful molecules.
Year:
2016
Link:
Study 2
Study type:
In-vitro study (cellular study)
Purpose:
To investigate whether the antioxidant properties (the ability to neutralise or counteract harmful molecules known as free radicals) of chaga mushroom extract can help to reduce DNA damage in immune cells called lymphocytes. Lymphocytes are a type of white blood cell that plays a crucial role in our immune system. The lymphocytes for this study were taken from both healthy individuals and individuals with inflammatory bowel disease.
Dose:
3 concentrations of chaga mushroom extract were used: 50, 100 and 500 micrograms of chaga mushroom extract per mL of water
Additional intervention:
50 micrograms/ml of hydrogen peroxide were added to the cells 30 minutes after chaga mushroom treatment
Results:
Chaga mushroom extract reduced DNA damage in the lymphocytes. In the patient group, there was a significant 54.9% reduction in DNA damage, whilst there was a 34.9% reduction in the control group. These findings suggest that chaga extract may have the potential to be a valuable supplement for inhibiting oxidative stress in general. Inhibiting oxidative stress is generally beneficial because excessive oxidative stress can damage cells, proteins, and DNA, leading to various diseases and accelerating the ageing process.
Year:
2007
Link:
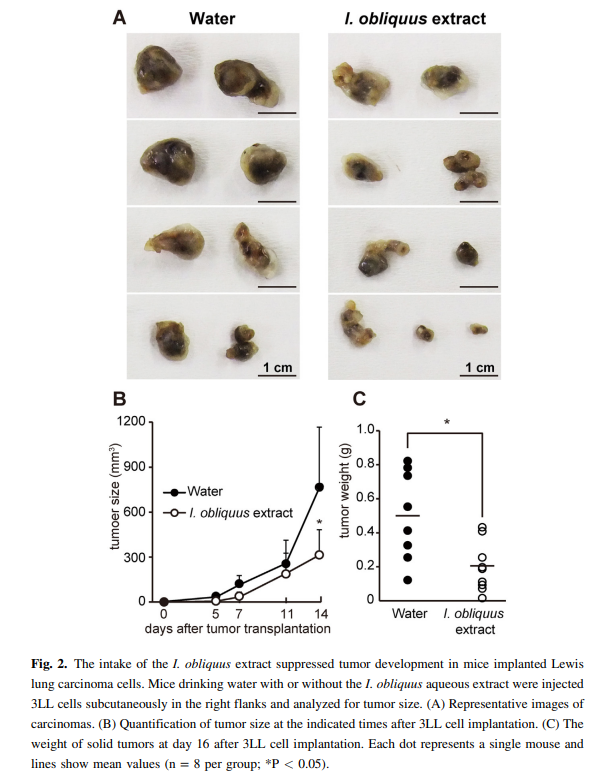
Study 1
Study type:
Rodent study
Purpose:
To investigate the effects of chaga mushroom extract on tumour suppression in mice.
Dose:
6 mg/kg/day of chaga mushroom extract in 5 ml of drinking water
Duration:
3 weeks prior to and 16 days after cancer cell implantation (intentionally introducing cancer cells into an organism).
Results:
The mice treated with chaga mushroom showed a significant delay in the growth of tumours. This effect was observed starting from day 14 after the cancer cells were introduced into the mice. By day 16, the average tumour size in the mice treated with chaga mushroom was 60.3% smaller than in the control group.
Additionally, the study observed that mice treated with chaga mushroom showed an increase in tumour agglomeration, which is a tendency for tumours to cluster together. This clustering may lead to more localised and concentrated tumour growth, as opposed to spreading widely to different parts of the body. Moreover, the inhibition of vascularisation (the formation of new blood vessels) observed in the chaga mushroom treated mice suggests that the formation of blood vessels necessary for tumour growth was reduced or prevented. Inhibiting vascularization can be a therapeutic strategy to stop tumour growth and prevent its spread to other parts of the body.
Year:
2008
Link:
Study 2
Study type:
Rodent study
Purpose:
To explore the effects of chaga mushroom polysaccharides on colitis-associated cancer in mice. Colitis-associated cancer refers to a type of cancer that develops in the colon (large intestine) as a result of long-standing chronic inflammation caused by colitis.
Dose:
150 mg/kg of chaga mushroom polysaccharides (force-fed every other day by inserting a tube into the stomach)
Duration:
101 days
Results:
Mice administered with chaga mushroom experienced less body weight loss, a higher survival rate, and longer colon length. Notably, the number of tumours in the colons of mice increased 80% in the control group compared to the 45% in the group treated with chaga mushroom. These findings suggest that chaga mushroom polysaccharides may be a therapeutic drug candidate for colitis-associated cancer.
Year:
2020
Link:
Study 3
Study type:
Rodent study
Purpose:
To investigate the antitumor effects of chaga mushroom in mice with tumours derived from human stomach cancer.
Dose:
50, 75, and 100 mg/kg/day oral administration of chaga mushroom polysaccharide
Duration:
10 days
Results:
Chaga mushroom polysaccharide supplementation significantly inhibited the growth of tumours in mice. It also increased the concentration of a protein called TNF-alpha that has anti-tumor activity. These findings suggest that chaga mushroom polysaccharides may have potential as an anti-tumor agent and may modulate the immune response against tumours.
Year:
2012
Link:
Study 4
Study type:
Cellular (in-vitro) and Rodent study (in-vivo)
Purpose:
To explore the mechanisms underlying the anti-cancer action of chaga mushroom polysaccharides in cellular and mice models.
Dose:
Cellular study: 0.1 - 1 mg/mL of chaga mushroom polysaccharides
Rodent study: 50 mg/kg of chaga mushroom polysaccharides or control (salt solution)
Results:
In the cellular experiment, chaga mushroom polysaccharide extract was found to activate an enzyme called AMPK (AMP-activated protein kinase) in lung cancer cells. This activation resulted in a decrease in cell growth and triggered cell death. These effects are important in combating cancer as they eliminate cancer cells and hinder tumour growth. Animal experiments further supported these findings, showing that treatment with chaga mushroom extract inhibited tumour growth and increased cell death. These results indicate that chaga mushroom polysaccharides have the potential to be a promising alternative or supplementary treatment option for cancer therapy.
Year:
2020
Link:
Study 5
Study type:
Cellular (in-vitro) and Rodent study (in-vivo)
Purpose:
To investigate the effects of ergosterol peroxide, a compound found in chaga mushroom, in mice with colitis-induced colorectal cancer cells.
Dose:
Cellular study: 10 or 40 μg/mL of ergosterol peroxide per week (5 or 20 μg/mL ergosterol peroxide x 2 per week)
Rodent study: 30 mg/kg/day of ergosterol peroxide (2 x 15 mg/kg of ergosterol peroxide administered by oral gavage (delivering the substance into the stomach through a tube inserted into the mouth), using a liquid solution)
Results:
The results from the cellular study demonstrated that long-term treatment with ergosterol peroxide derived from chaga mushroom at concentrations of 10 μg/mL and 5 μg/mL significantly decreased the formation of cell clusters (colonies) in colorectal cancer cells. This suggests that ergosterol peroxide has the potential to impede the growth of cancer cells. In the case of one specific type of cancer cell from the human colon (HT-29), a concentration of 5 μg/mL completely halted the formation of these cell clusters. These findings contribute additional evidence supporting the anticancer effects of ergosterol peroxide.
In addition, ergosterol peroxide administration suppressed tumour growth and reduced total tumour count in both prevention and therapy groups in mice with colitis-induced colorectal cancer cells. Furthermore, it also decreased the number of both small and large tumours in mice.
Year:
2015
Link:
Study 1
Study type:
Rodent study
Purpose:
To evaluate the effects of chaga mushroom polysaccharide in diabetic mice.
Dose:
0.4, 0.8, 1.2 g/kg/day of chaga mushroom polysaccharide or 0.25 g/kg of Metformin (a prescription medication that is commonly used to treat type 2 diabetes)
Duration:
4 weeks
Results:
High oral doses of chaga mushroom polysaccharide (1.2 g/kg/day) resulted in a significant 31% reduction in blood sugar levels in mice with diabetes.
Chaga mushroom polysaccharide also improved abnormal lipid levels in the blood of diabetic mice. When the levels of fats (lipids) in the blood are abnormal, such as having too much “unfavourable” cholesterol (LDL-cholesterol) or triglycerides, it can lead to the buildup of fatty deposits in the arteries. These deposits can block or narrow the blood vessels, which can increase the risk of heart disease and stroke.
Overall, the findings suggest that chaga mushroom polysaccharide holds potential as a therapeutic agent for managing blood sugar levels and improving lipid profiles in individuals with diabetes.
Year:
2021
Link:
Study 2
Study type:
Rodent study
Purpose:
To investigate the anti-diabetic effects of chaga mushroom in mice with diabetes.
Dose:
300 mg/kg/day, 600 mg/kg/day, and 900 mg/kg/day of chaga mushroom polysaccharides-chromium (III) complex
Duration:
4 weeks
Results:
4 weeks of supplementation with chaga mushroom polysaccharides led to significant reductions in body weight, blood sugar levels, and insulin levels in diabetic mice compared to untreated diabetic mice. Chaga mushroom polysaccharides effectively reduced cellular damage caused by diabetes in mice. The study did not find any harmful effects of high doses of chaga mushroom polysaccharides in healthy mice, and their organs remained healthy. These findings suggest that chaga mushroom polysaccharides could be a good option for treating type 2 diabetes.
Year:
2017
Link:
Study 3
Study type:
Rodent study
Purpose:
To investigate the effects of chaga mushroom extract in mice with type 2 diabetes.
Dose:
30 and 60 mg/kg/day of chaga mushroom or 25 mg/kg/day of glibenclamide (a commercial oral medication used in the management of type 2 diabetes) or control
Duration:
21 days
Results:
Chaga mushroom treatment for 3 weeks reduced blood sugar levels in mice. After 7 days of treatment, the extract lowered blood glucose levels by 11.54% and 15.15%, respectively, and after 21 days, the reduction increased to 22.51% and 24.32%.
In comparison, the group treated with the diabetes medication glibenclamide showed a decrease of 21.87% and 36.71% in their blood glucose levels after 7 and 21 days, respectively. These findings suggest that chaga mushroom has a beneficial impact on lowering blood glucose levels in diabetic mice, although the reduction is not as significant as that achieved with glibenclamide.
Year:
2010
Link:
Study 4
Study type:
Rodent study
Purpose:
To explore the effects of chaga mushroom extract on the intestinal flora of mice with type 2 diabetes.
Dose:
600 mg/kg/day of chaga mushroom extract
Duration:
8 weeks
Results:
In mice treated with chaga mushroom, researchers observed reduced blood sugar, reduced inflammation and reduced levels of lipids in the blood. Lipids, such as cholesterol and triglycerides, are fatty substances that can build up in the bloodstream and contribute to various health issues, including cardiovascular diseases.
The study also showed that chaga mushroom extract had potential benefits for type 2 diabetes by increasing beneficial gut bacteria and reducing harmful bacteria. People with type 2 diabetes tend to have fewer good bacteria in their gut, which is being explored as a possible factor in the disease.
Overall, the findings suggest that chaga mushroom has potential as a therapeutic agent for managing diabetes and promoting gut health.
Year:
2022
Link:
Study 5
Study type:
Rodent study
Purpose:
To investigate the anti- diabetic effects of chaga mushroom extract in mice with type 2 diabetes
Dose:
100, 250, and 500 mg/kg of chaga mushroom extract or 10 mg/kg Metformin (a prescription medication that is commonly used to treat type 2 diabetes), or a control (salt solution)
Results:
Oral administration of doses of 250 mg/kg and 500 mg/kg of chaga mushroom extract significantly improved blood sugar levels and insulin resistance in mice. Chaga mushroom also increased liver glycogen content. Increased glycogen content can be beneficial as it provides a readily available source of energy for the body during times of increased physical activity or when blood glucose levels are low.
Furthermore, the study found increased levels of HDL-cholesterol (“favourable” cholesterol), while reducing LDL-cholesterol (“unfavourable” cholesterol) levels, triglycerides, and total cholesterol. Higher levels of HDL-cholesterol may be beneficial for cardiovascular health, while lower LDL-C and triglycerides levels are associated with a reduced risk of heart disease.
Overall, the results suggest that chaga mushroom extract has the potential to lower blood sugar and improve lipid levels, making it potentially beneficial for managing type 2 diabetes.
Year:
2021
Link:
Study 6
Study type:
Rodent study
Purpose:
To assess the anti-diabetic effects of chaga mushroom extract in mice with type 2 diabetes. Diabetes was induced in the mice by a high-fat diet combined with streptozotocin, a naturally occurring chemical compound.
Dose:
150 mg/kg of chaga mushroom extract
Duration:
5 weeks
Results:
The study demonstrated that the administration of chaga mushroom extract had significant benefits in reducing high blood sugar levels in mice. Additionally, treatment with chaga mushroom resulted in significant improvements in blood lipid levels, glucose tolerance, and insulin resistance in diabetic mice. This means that the levels of fats in the blood, the ability to regulate blood sugar levels, and the body's response to insulin were all positively affected, leading to better overall metabolic health and a reduced risk of developing type 2 diabetes.
Notably, there was a significant increase in HDL cholesterol (high-density lipoprotein cholesterol) following chaga mushroom administration. Higher levels of HDL are generally associated with a reduced risk of heart disease and other cardiovascular complications in individuals with diabetes.
Overall, these findings suggest that chaga mushroom has the potential to be a beneficial dietary intervention for managing high blood glucose levels and improving metabolic parameters in diabetes, particularly by positively impacting blood lipid profiles and increasing HDL cholesterol levels.
Year:
2022
Link:
Study 7
Study type:
Rodent study
Purpose:
To investigate the effects of chaga mushroom polysaccharides in mice with diabetes.
Dose:
50 mg /kg of chaga mushroom polysaccharides
Duration:
4 weeks
Results:
The findings revealed that chaga mushroom polysaccharides had positive effects on diabetes. Chaga mushroom polysaccharides increased insulin levels in the mice and improved the synthesis of glycogen, which helps regulate blood sugar levels. Chaga mushroom polysaccharides also restored the balance of antioxidants in the body. Antioxidants are crucial in diabetes management, as they counteract inflammation and oxidative stress caused by harmful molecules called free radicals.
In addition, chaga mushroom polysaccharides exhibited anti-inflammatory effects by reducing the levels of specific molecules associated with inflammation. It also reduced the expression of a molecule called phosphor-NF-κB in the kidneys, which indicates a decrease in inflammation. Several studies have found that chronic inflammation is linked to high blood sugar and contributes to insulin resistance in type 1 diabetes.
Overall, the results suggest that chaga mushroom extracts may help protect against diabetes-related kidney damage by restoring antioxidants and reducing inflammation.
Year:
2017
Link:
Study 8
Study type:
Rodent study
Purpose:
To investigate the potential protective effects of trametenolic acid, one of the active compounds found in chaga mushrooms, in mice with diabetic kidney disease (diabetic nephropathy).
Dose:
10 mg/kg/day of trametenolic acid (injected into the lower abdominal area) or control (salt solution)
Duration:
4 weeks
Results:
The study found that administration of trametenolic acid, derived from chaga mushrooms, had beneficial effects in mice with diabetic kidney disease. The results also showed that trametenolic acid treatment reduced the ratio of right kidney weight to body weight.The decrease in this ratio suggests that trametenolic acid treatment potentially reduced inflammation, fluid accumulation, and promoted kidney health. In diabetic kidney disease, the kidneys can enlarge due to fluid retention.
Furthermore, trametenolic acid from chaga mushrooms increased the levels of protective substances that defend against cell damage while reducing the levels of a harmful substance associated with oxidative stress. Additionally, trametenolic acid decreased the levels of inflammatory molecules that could harm the kidneys. It also improved the expression of essential proteins for kidney function and lowered the levels of proteins associated with kidney scarring.
These findings suggest that trametenolic acid has a protective effect on the kidneys in diabetic kidney disease. It helped alleviate oxidative stress, inflammation, and kidney damage, providing a potential treatment approach for this condition.
Year:
2022
Link:
Study 9
Study type:
Rodent study
Purpose:
To evaluate the effects of chaga mushroom in mice with diabetes.
Dose:
500 and 1000 mg/kg/day of chaga mushroom
Duration:
3 weeks
Results:
The study found that chaga mushroom has significant benefits for mice with diabetes. When treated with chaga mushroom, the mice experienced lower blood sugar levels and increased antioxidant activity. Antioxidants are important in managing diabetes as they can enhance insulin sensitivity and reduce oxidative stress. Oxidative stress is characterised by an excess of unstable molecules called free radicals in the body, and not enough antioxidants to get rid of them, resulting in cellular damage.
Additionally, the lipid (fats) balance in the mice was improved, as seen through a reduction in free fatty acids, total cholesterol, triglycerides, and LDL-cholesterol (“unfavourable” cholesterol), and an increase in HDL-cholesterol (“favourable” cholesterol). These changes are associated with a reduced cardiovascular risk and lower chance of diabetes complications.
Chaga mushroom also increased insulin levels and restored the health of the pancreas (the organ where insulin is produced). These findings suggest that chaga mushroom could be a potential treatment for diabetes by improving blood sugar and protecting against complications associated with the disease.
Year:
2008
Link:
Study 10
Study type:
Rodent study
Purpose:
To investigate the antidiabetic activity of chaga mushroom polysaccharides, particularly its hypoglycemic effects (its ability to lower blood sugar) and lipid-lowering effects (its ability to lower blood cholesterol) in mice with type 2 diabetes. It is important to understand that these hypoglycemic and lipid-lowering effects are crucial for managing diabetes effectively, preventing complications, and promoting better overall health in individuals with diabetes.
Dose:
150 mg/mL, 300 mg/mL, and 600 mg/mL/day of chaga mushroom polysaccharides
Duration:
1 week of chaga mushroom polysaccharide treatment
Results:
Chaga mushroom polysaccharides had protective effects against type 2 diabetes in mice. This was achieved by strengthening the intestine's natural defence system, which acts as a barrier to prevent harmful substances like toxins or bacteria from entering the bloodstream and causing harm to the body.
Additionally, treatment with chaga mushroom resulted in changes in the gut bacteria composition, specifically an increase in Firmicutes bacteria, which play various roles in our bodies, including aiding in digestion, supporting the immune system, and producing certain vitamins. The administration of chaga mushroom polysaccharides also showed a dose-dependent effect in reducing blood sugar levels in the mice.
Overall, the findings of the study suggest that chaga mushroom holds promise as a potential novel treatment for type 2 diabetes.
Year:
2022
Link:
Study 1
Study type:
Rodent study
Purpose:
To investigate the effects of chaga mushroom extract in mice with liver injuries.
Dose:
200 mg/kg/day of orally administered chaga mushroom extract or control (saltwater solution)
Duration:
8 weeks (4 weeks of chaga mushroom treatment)
Results:
The study revealed that microcystin-leucine arginine exposure, a specific type of toxin which can cause liver damage in mice, caused a significant decrease in the body weight of mice. However, treatment with chaga mushroom extract restored their body weight to a level similar to the control group. The mice exposed to microcystin-leucine arginine also showed elevated levels of liver damage markers which indicate potential liver dysfunction or injury. When treated with chaga mushroom extract, the levels of these markers returned to normal, indicating healthy liver function.
Furthermore, the microcystin-leucine arginine-exposed mice exhibited reduced levels of glutathione, an important antioxidant. However, chaga mushroom treatment effectively restored the glutathione levels to normal. Lower levels of glutathione indicate a decrease in the body's ability to protect cells from damage.
Year:
2022
Link:
Study 2
Study type:
Rodent study
Purpose:
To explore the protective effects and mechanism of chaga mushroom polysaccharide in mice with liver injury caused by Toxoplasma gondii infection. It is important to note that the liver is an important site for the reproduction of the parasite Toxoplasma gondii and this process can lead to changes in liver function and disrupt its normal activities.
Dose:
100, 200, and 400 mg/kg of chaga mushroom polysaccharide or 200 mg/kg of Sulfadiazine (a widely used medicine for toxoplasmosis, which is a parasitic infection with Toxoplasma gondii) or control (saltwater solution)
Duration:
7 days
Results:
The results of the study indicate that chaga mushroom polysaccharides have protective effects in mice with liver injury caused by Toxoplasma gondii. Treatment with chaga mushroom led to a decrease in liver damage and oxidative stress markers. A decrease in oxidative stress markers indicates a reduction in the level of damage caused by harmful molecules in the body, suggesting a more balanced and healthier state. Additionally, chaga mushroom polysaccharides improved liver damage caused by Toxoplasma gondii and reduced the levels of inflammatory cytokines, which are proteins that help coordinate immune responses and inflammation. This reduction in inflammatory cytokines signifies a decrease in inflammation.
Overall, chaga mushroom polysaccharides show potential to protect the liver against damage caused by Toxoplasma gondii.
Year:
2019
Link:
Study 3
Study type:
Rodent study
Purpose:
To evaluate the effects of chaga mushroom and its compounds in mice with non-alcoholic fatty liver disease (NAFLD). NAFLD is commonly associated with factors like obesity, insulin resistance, metabolic syndrome, and poor dietary habits.
Dose:
100 and 200 mg/kg/day of chaga mushroom or control (normal chow diet, , which refers to the standard diet given to laboratory rodents)
Results:
The study revealed that chaga mushroom and its active compounds, including inotodiol, lanosterol, and trametenolic acid, had beneficial effects in reducing fat buildup in the livers of the mice. These compounds also reduced the activity of genes responsible for producing fat. Since excessive fat accumulation in the liver can lead to inflammation and liver damage, the findings suggest that chaga mushroom and its compounds hold promise as potential treatments for non-alcoholic fatty liver disease (NAFLD) by targeting specific molecular pathways involved in fat metabolism.
Year:
2022
Link:
Study 1
Study type:
Rodent study
Purpose:
To investigate the cardioprotective effect of chaga mushroom extract against myocardial ischemia/reperfusion injury in rats. Myocardial ischemia/reperfusion injury refers to the damage that occurs to the heart muscle when blood flow is temporarily restricted (ischemia) and then restored (reperfusion).
Dose:
150, 300, and 600 mg/kg of chaga mushroom extract
Duration:
7 days
Results:
The study demonstrated that treatment with chaga mushroom extract improved cardiac function and reduced the size of heart tissue damage (infarct size). Additionally, pretreatment with chaga mushroom extract increased the activity of protective enzymes and the production of a protein called SIRT1, which helps regulate cell health. It also reduced the levels of proteins associated with cellular stress and cell death. Chaga mushroom extract also prevented cell death caused by stress in heart muscle cells (cardiomyocytes). Reducing cell death in the heart tissue can be beneficial for maintaining heart health and preventing heart damage. These findings indicate that chaga mushroom extract has the potential to be a treatment option for cardiovascular diseases.
Year:
2021
Link:
Study 1
Study type:
Rodent study
Purpose:
To investigate the effects of chaga mushroom polysaccharides in mice with colitis (a condition where the colon becomes inflamed, leading to symptoms such as abdominal pain, diarrhoea, and sometimes rectal bleeding).
Dose:
100, 200, 300 mg/kg/day of chaga mushroom polysaccharides
Duration:
43 days
Results:
In mice with colitis, chaga mushroom polysaccharides prevented weight loss, reduced rectal bleeding, and improved stool consistency compared to the control group.
Additionally, the mice in the control group exhibited a substantial reduction in colon length by 6.8 cm compared to the normal group, indicating the presence of colitis. A decrease in colon length is a prominent characteristic of colitis, representing inflammation and damage to the colon tissue. However, treatment with chaga mushroom alleviated this colon-shortening effect.
Year:
2019
Link:
Study 2
Study type:
Rodent study
Purpose:
To investigate the effects of chaga mushroom polysaccharide on gut microbiota (the community of microorganisms that live in the digestive system) in mice with chronic pancreatitis. Pancreatitis is a condition where the pancreas, an organ in the abdomen, becomes inflamed or swollen.
Dose:
0.1, 0.2, and 0.4 g/kg/day of chaga mushroom polysaccharide or 3.7 g/kg/day Qingyilidan (a commercial medicine in China) or control (saline/saltwater solution)
Duration:
5 weeks
Results:
Chaga mushroom polysaccharide treatment had positive effects in mice with chronic pancreatitis. The treatment increased the overall antioxidant capacity in mice and lowered the levels of certain molecules that are involved in inflammation, such as tumour necrosis factor alpha (TNF-α) and transforming growth factor beta (TGF-β). Increased levels of these molecules promote inflammation and can contribute to pancreas damage.
The study also found that chaga mushroom treatment regulated the gut microbiota towards a healthier profile. This means that the chaga mushroom helped restore the balance of different types of bacteria in the gut, which is crucial for overall health.
The positive changes in both the biochemical markers and the gut bacteria suggest that chaga mushroom treatment could be effective in improving chronic pancreatitis and gut microbiota.
Year:
2017
Link:
Study 3
Study type:
Rodent study
Purpose:
To investigate the effects of chaga mushroom on acute colitis (inflammation of the colon) in mice.
Dose:
100 and 200 mg/kg body weight of chaga mushroom extract
Duration:
14 days
Results:
The study demonstrated that chaga mushroom extract had beneficial effects in mice with acute colitis: it reduced inflammation, protected the intestinal lining, and prevented damage to important structures in the intestines. It also improved markers of inflammation severity, lowered levels of inflammatory enzymes, and decreased the presence of chemicals that promote inflammation. These findings suggest that chaga mushroom extract holds promise for potential clinical use in treating inflammatory colitis.
Year:
2012
Link:
Study 4
Study type:
Rodent study
Purpose:
To evaluate the effects of chaga mushroom polysaccharide on the gut microbiota in mice and ulcerative colitis. Ulcerative colitis is a condition that causes inflammation and ulcers in the colon and rectum, leading to symptoms like abdominal pain and bloody diarrhoea.
Dose:
100, 200 and 400 mg/kg/day of chaga mushroom polysaccharide or controls (1.08 g/kg/day of commercial medicine for ulcerative colitis or a salt solution)
Duration:
1 week
Results:
The mice treated with chaga mushroom polysaccharides experienced an increase in superoxide dismutase activity, which indicates a positive impact on the body's antioxidant defences. Antioxidant defences help protect our cells from damage caused by harmful molecules called free radicals.
Furthermore, chaga mushroom supplementation reduced the secretion of substances such as interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumour necrosis factor-α (TNF-α). The reduction in the secretion of these substances indicates a decrease in inflammation, suggesting that chaga mushroom has anti-inflammatory properties.
Year:
2023
Link:
Study 5
Study type:
Rodent study
Purpose:
To investigate the effects of chaga mushroom polysaccharide on the intestinal flora in mice with endometritis (inflammation or infection of the lining of the uterus/endometrium).
Dose:
150 mg/kg of chaga mushroom polysaccharide (or a control (saline/saltwater solution)
Results:
The study found that when mouse uterine cells were exposed to a substance called lipopolysaccharide (LPS), they became swollen and had an increased presence of eosinophils (immune cells associated with inflammation). However, when the cells were treated with chaga mushroom, these changes were significantly improved, suggesting that chaga mushroom has a positive effect on reducing inflammation in the uterus. Additionally, the levels of certain inflammatory cytokines (IL-6, IL-1β, and TNF-α) decreased in the chaga mushroom treatment group. Inflammatory cytokines are small proteins produced by different cells in the body that are released when the immune system responds to an infection or injury, causing inflammation. A decrease in inflammatory cytokines means a reduction in the levels or activity of these substances that promote inflammation. This suggests that chaga mushroom polysaccharide could potentially help alleviate the symptoms of endometritis, an inflammatory condition.
Year:
2021
Link:
Study 1
Study type:
Rodent study
Purpose:
To investigate whether chaga mushroom extract can inhibit antibody production and a severe allergic reaction in mice. Allergies occur when the immune system produces too many antibodies in response to harmless substances, leading to the release of inflammatory substances like histamine, resulting in symptoms like itching, sneezing, or hives. Inhibiting antibody production is crucial in managing allergies as it helps decrease the exaggerated immune response and alleviate the associated symptoms.
Dose:
0.1, 1, and 10 mg/day of chaga mushroom extract dissolved
in water
Duration:
21 days
Results:
The study found that chaga mushroom extract could help prevent a severe allergic reaction called systemic anaphylactic shock in mice.
Chaga mushroom administration significantly reduced the levels of a specific type of antibody called IgE (Immunoglobulin E), which is associated with allergies. Since IgE is closely associated with allergic reactions, this reduction suggests that the Chaga mushroom extract may have a suppressive effect on the immune response involved in allergies. This effect is beneficial as it helps regulate and lessen the exaggerated immune response that causes allergy symptoms, leading to potential relief from allergies.
Additionally, when spleen cells from the mice were exposed to a substance called ovalbumin, those treated with chaga mushroom extract exhibited an increased production of a protein called IFN-γ (interferon-gamma), indicating a boost in immune responses. IFN-γ is generally associated with suppressing allergic reactions and can inhibit the production of IgE antibodies.
These results suggest that chaga mushroom extract has the potential to be used as a type of food that could help prevent allergies.
Year:
2013
Link:
Study 2
Study type:
Rodent study
Purpose:
To investigate the anti-allergic activities of chaga mushroom in mice with allergies.
Dose:
50, 100, or 200 mg/kg/day of chaga mushroom extract or 0.1 mL of salt solution (control)
Duration:
11 days (5 consecutive days of treatment, starting on day 7 of the experiment)
Results:
Chaga mushroom extract regulated the balance between proteins called Th1 and Th2 cytokines. Achieving a proper balance between Th1 and Th2 cytokines is crucial for maintaining a well-functioning immune system and protection against various pathogens.
Furthermore, the study found that chaga mushroom extract reduced the production of nitric oxide and tumour necrosis factor, which indicates a potential reduction in inflammation.
Overall, the results suggest that chaga mushroom extract may have anti-allergic and anti-inflammatory properties, making it a potential option for managing allergic reactions and inflammation.
Year:
2011
Link:
Study 3
Study type:
Rodent study
Purpose:
To compare the anti-allergic effects between inotodiol, a compound found in chaga mushroom, and raw chaga mushroom in mice with food allergies.
Dose:
20 mg/kg of inotodiol or 320 mg/kg of raw chaga mushroom or 10 mg/kg of Dexamethasone (a common medication to reduce inflammation and suppress the immune system)
Duration:
1-4 days
Results:
In mice with food allergies, inotodiol had a significant positive impact on allergy symptoms and inflammation in the small intestine.
Inotodiol specifically targeted and inhibited the function of a type of cell called mast cells. When mast cell function is inhibited, it can lead to a decrease in the release of inflammatory mediators (substances that promote and regulate the inflammation process) such as histamine, which can help alleviate allergic symptoms and reduce inflammation. The strong anti-allergic effects and selective action of inotodiol on mast cells make it a promising therapeutic option for food allergies.
Year:
2020
Link:
Study 1
Study type:
Rodent study
Purpose:
To assess the effects of chaga mushroom extract in obese mice.
Dose:
1000 mg/kg/day of chaga mushroom extract or control (water)
Additional intervention:
A high fat diet consisting of 18% fat, 20% carbohydrate, 3% egg, and 59% normal diet
Duration:
12 weeks
Results:
Chaga mushroom extract for 12 weeks reduced the accumulation of fat in the body. This is accompanied by lowered levels of free fatty acids, triglycerides, and total cholesterol in the blood, liver, and fat tissue. Obesity is often associated with increased levels of these lipid markers, which can lead to health issues like insulin resistance and cardiovascular disease. Therefore, the reduction in these lipid markers suggests improved lipid regulation and may be beneficial for managing obesity-related health risks. Overall, chaga mushroom extract shows promise as a potential anti-obesity agent.
Year:
2015
Link:
PRODUCTS CONTAINING CHAGA
Chaga Capsules

Cordyceps Scientific Studies
Numerous clinical trials and animal studies have investigated the effects of cordyceps. We have summarised the most interesting results related to immune system, performance enhancement, kidney health, fatigue, testosterone, sexual function, and diabetes.
Study 1
Study type:
Randomised, double-blinded, placebo-controlled, prospective clinical trial
Purpose:
To assess the immune-enhancing effects of Cordyceps militaris in healthy male adults.
Dose:
1.5 g/day of Cordyceps militaris or placebo
Participants:
80 healthy men aged 19-64 years
Duration:
4 weeks
Results:
Cordyceps militaris supplementation had positive effects on the immune system of healthy adult males. Participants who took a daily dose of 1.5 g of Cordyceps militaris showed increased levels of cytokines, which are small proteins that regulate the immune response. Specifically, it increased the number of lymphocytes (a type of white blood cell that is part of the immune system and elevated levels of T-helper cell 1 cytokines, which enhance immune responses. Notably, Cordyceps militaris was found to be safe with no reported side effects.
Year:
2015
Link:
Study 2
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To investigate the efficacy and safety of a cordyceps mycelium extract in healthy Korean adults.
Dose:
1.68 g/day of Cordyceps mycelium culture extract (2 x 1.34 g capsules taken after breakfast and dinner) or placebo
Participants:
79 males and females, aged 20-75 years
Duration:
8 weeks
Results:
Cordyceps mycelium supplementation was associated with a significant increase in natural killer cells (21.2% to 56.4%). Natural killer cells are immune cells that are crucial for protecting the body against viral infections and cancer, and an increase in natural killer cell levels signifies a stronger immune response and enhanced ability to fight infections and diseases. Although slight increases were observed in other cytokines (small proteins involved in regulating immune responses) like TNF-α and IL-12, the differences were not statistically significant compared to the placebo group.
No serious adverse events were reported during the study period.
Year:
2019
Link:
Study 3
Study type:
Rodent study
Purpose:
To investigate the effect of Cordyceps militaris polypeptide on the immune function of immunosuppressed mice.
Dose:
32, 160, and 800 mg/kg/day of Cordyceps militaris polypeptide or control (distilled water)
Duration:
45 days
Results:
Cordyceps militaris polypeptide boosted the immune system in mice, increased the number of white blood cells and delayed allergy responses. An increase in serum hemolysin content was also observed, which suggests an enhancement of the immune system's ability to combat pathogens, as hemolysin plays a role in destroying harmful microorganisms and contributes to the body's immune response against infections. These findings suggest that Cordyceps militaris polypeptide has the potential to enhance immunity.
Year:
2018
Link:
Study 1
Study type:
Systematic review
Purpose:
To explore the anti-neuroinflammatory (the ability to reduce or counteract neuroinflammation, which is the inflammation of neural tissue in the brain or nervous system, often associated with various neurological disorders) activities of medicinal mushrooms, including Cordyceps in various laboratory studies and animal studies.
Results:
Cordyceps militaris is known for its bioactive compound, cordycepin. Numerous studies have highlighted cordycepin's positive effects, especially in the central nervous system (which consists of the brain and spinal cord), where it demonstrates anti-neuroinflammatory properties, meaning that they have properties that counteract inflammation in the brain and nervous system.
One rodent study revealed that cordycepin from Cordyceps militaris offers advantages against chronic unpredictable mild stress in mice with behavioural deficits. Other research found that it reduced depressive-like behaviours and decreased inflammation in mice. Further studies suggest that cordycepin, along with a hot water extract from Cordyceps militaris, has exhibited the ability to shield brain cells during reduced blood flow, reducing damage from harmful molecules and lowered the activation of certain brain cells, indicating neuroprotective effects.
One study on rodents demonstrated that cordycepin, derived from Cordyceps militaris, countered the effects of chronic unpredictable mild stress, improving the behaviour of the mice. It has also been found that cordycepin reduced depressive-like behaviours and decreased inflammation in mice.
Further studies found that cordycepin, in combination with a hot water extract from Cordyceps militaris, protected brain cells during periods of reduced blood flow, reducing damage from harmful molecules, indicating neuroprotective effects.
Year:
2020
Link:
Study 2
Study type:
Rodent study
Purpose:
To assess the anti-ageing effects of Cordyceps sinensis in aged and castrated mice.
Method of evaluation:
Learning and memory were assessed using water maze and step-down type avoidance tests. The water maze test measures spatial learning and memory in animals by requiring them to find a hidden platform in a pool of water using visual cues. The step-down type avoidance test evaluates learning and memory in rodents by measuring their ability to associate an elevated platform with a foot shock and subsequently avoid stepping down.
Sexual activity of male mice was estimated by copulation behaviour after a female rat in estrus (a specific stage in the reproductive cycle when mating and conception are most likely to occur) was placed into the male’s cage.
Penis erection was evaluated using a laboratory apparatus in which an electrode was placed on the penis of male mice, and continuous electric shocks were administered until the penis achieved an erection. The time taken from the shock application to the onset of erection was measured as the penis erection latency.
Dose:
1.0, 2.0, or 4.0 g/kg of Crodyceps sinensis extract or controls
Duration:
6 weeks
Results:
The study found that doses of 2.0 and 4.0 mg/kg of cordyceps sinensis extract significantly improved learning and memory in aged mice compared to normal controls, in both water maze test and step-down avoidance tests. Among mice treated with the highest dose (4.0 g/kg) of Cordyceps, the cellular structure in the hippocampus (a region within the brain responsible for memory and learning) remained well-preserved, with no signs of swelling. This observation suggests that cordyceps sinensis extract has a protective effect on the cellular structure of the hippocampus in aged mice, potentially contributing to its anti-ageing effect.
The study also found that cordyceps sinensis shortened penis erection latency (the time it takes for the penis to become erect in response to a stimulus) and mount latency (the time it takes for a male animal to mount a female for sexual activity) in castrated mice, indicating a positive impact on sexual function.
In addition, cordyceps sinensis extract improved the activity of antioxidative enzymes in aged mice, indicating its antioxidative effect. The study further discovered that the extract lowered the activity of monoamine oxidase (an enzyme that breaks down neurotransmitters in the brain) in aged mice, suggesting potential for enhanced brain function and prevention of age-related cognitive decline.
Overall, these findings suggest that cordyceps sinensis extract has a potential anti-ageing effect by improving brain function, antioxidative enzyme activity, and sexual function.
Year:
2011
Link:

Study 1
Study type:
Rodent study
Purpose:
To investigate the lifespan-extending effect of Cordyceps sinensis in normal mice.
Dose:
500, 1000, and 1500 mg/kg body weight of Cordyceps sinensis or control
Results:
All the control mice died before reaching 3 years of age, while the mice receiving Cordyceps sinensis had an extended lifespan. The extension in lifespan varied depending on the dose of Cordyceps sinensis, with an increase of 10-66 days when half of the mice survived (50% survival) and 45-153 days when only 10% of them survived.
The age of the oldest surviving mice was extended by 154 and 258 days at 500 mg/kg and 1500 mg/kg doses, respectively, after Cordyceps sinensis treatment. The highest effect was observed at a dose of 1000 mg/kg, which was extended by 354 days.
Year:
2011
Link:
Study 2
Study type:
Fruit fly study
Purpose:
To investigate the effects of Cordyceps sinensis on the lifespan of fruit flies.
Dose:
0.02, 0.06, 0.20 mg/ml of Cordyceps sinensis oral liquid or control
Duration:
55 days
Results:
The results showed that lifelong treatment with Cordyceps sinensis significantly prolonged the lifespan of fruit flies. Fruit flies typically live 40-50 days, but the average lifespan of the fruit flies was extended by 44, 46 and 47 days with Cordyceps sinensis at doses of 0.02, 0.06, 0.20 mg/ml, respectively. The maximum lifespan (the average lifespan of the longest surviving 10% of fruit flies) was extended by 67-69 days with the highest effect observed at the 0.06 mg/ml dose (69 days).
Year:
2015
Link:
Study 1
Study type:
Randomised, double-blinded, placebo-controlled clinical trial
Purpose:
To determine the effects of Cordyceps militaris on high intensity exercise in healthy adults
Dose:
4 g/day of mushroom blend containing Cordyceps militaris (3 x 1.3 g capsules) or placebo (4 g of maltodextrin)
Participants:
28 recreationally active adults aged 18-35 years for Phase 1 (week 1); 10 volunteered to complete phase II (additional 2 weeks).
Duration:
1-3 weeks
Results:
The study revealed that one week of supplementation with Cordyceps militaris did not lead to significant improvements in performance compared to the placebo. However, during a three-week period of taking the supplements, the researchers noticed that the participants' maximal oxygen consumption improved. This means that their aerobic capacity, or their body's ability to perform endurance activities, improved.
There were also indications of potential improvements in the ventilatory threshold and time to exhaustion, indicating improved endurance and the ability to sustain higher exercise intensities for a longer duration. These findings suggest that longer-term or chronic supplementation with Cordyceps militaris may benefit exercise performance.
Year:
2016
Link:
Study 2
Study type:
Double-blinded, placebo-controlled, prospective clinical trial
Purpose:
To examine the effect of cordyceps sinensis on exercise performance in healthy elderly subjects.
Dose:
999 mg/day of cordyceps sinensis (3 x 333 mg capsules) or placebo
Participants:
20 males and females, aged 50-75 years
Duration:
12 weeks
Results:
After 12 weeks of cordyceps sinensis supplementation, there was a significant 10.5% increase in the metabolic threshold and an 8.5% increase in the ventilatory threshold. The metabolic threshold represents the point during exercise when the body starts using more anaerobic energy due to insufficient oxygen supply, leading to fatigue. The ventilatory threshold represents the point when the breathing rate increases to meet the increased oxygen demands of the muscles, allowing for better endurance during exercise. These improvements in both thresholds indicate an enhanced ability to sustain higher levels of exercise intensity.
These improvements were not observed in the placebo group, suggesting that cordyceps sinensis supplementation specifically contributed to the enhanced thresholds. On the other hand, no notable changes were observed in the maximum oxygen uptake, maximum heart rate, maximum work rate, or maximum ventilation in either group when compared to baseline.
Year:
2010
Link:

Study 3
Study type:
Rodent study
Purpose:
To evaluate the effect of Cordyceps sinensis supplementation on exercise endurance of rats.
Dose:
200 mg/kg body weight of Cordyceps sinensis mycelia powder or control
Additional intervention:
The exercise groups were subjected to swimming training for 15 days (6 days a week)
Duration:
15 days
Results:
Cordyceps sinensis treatment with and without exercise significantly improved swimming endurance in rats, by 2.9-fold in rats who exercised and 1.79-fold in rats who did not exercise, compared to placebo rats. Additionally, the study found that treatment with Cordyceps sinensis resulted in increased expression of skeletal muscle metabolic regulators. These regulators are proteins and enzymes that help regulate important metabolic processes within muscle cells, indicating an enhancement in the metabolic activity of the muscles. These findings suggest that Cordyceps sinensis supplementation can improve muscle performance and metabolic regulation, even without exercise, providing molecular evidence of its beneficial effects.
Year:
2011
Link:

Study 1
Study type:
Rodent study
Purpose:
To investigate the effect Cordyceps militaris polysaccharide on physical fatigue induced in mice through a forced swimming test.
Method of evaluation:
The mice in all groups underwent the forced swimming test 30 minutes after the final administration of Cordyceps militaris. The mice were considered fatigued when they were unable to reach the water surface to breathe within a 7-second period, which served as an indicator of swimming capacity.
Dose:
40, 80, 160 mg/kg body weight/day of Cordyceps militaris polysaccharide or control (drinking water)
Duration:
28 days
Results:
The study found that Cordyceps militaris polysaccharide has anti-fatigue properties and can lower serum urea nitrogen (UN) levels. During intense exercise, the body breaks down proteins and amino acids to produce energy, which increases UN levels. However, the study found that Cordyceps militaris polysaccharide can reduce the breakdown of proteins, leading to lower UN levels. This suggests that Cordyceps militaris polysaccharide may delay physical fatigue by preserving proteins and slowing down the onset of exhaustion.
Additionally, Mice treated with Cordyceps militaris polysaccharide at doses of 40, 80, and 160 mg/kg body weight exhibited significantly longer exhaustive swimming times, with increases of 29.81%, 45.22%, and 70.39% respectively compared to the control group.
These findings indicate that Cordyceps militaris polysaccharide holds potential as a new functional food or medicine for combating fatigue.
Year:
2016
Link:
Study 2
Study type:
Rodent study
Purpose:
To investigate the effects of cordycepin (a principal active ingredient from Cordyceps militaris) on physical fatigue in mice.
Dose:
20 and 40 mg/kg of cordycepin or 500 mg/kg of taurine (a commonly used dietary supplement to relieve fatigue) or control (saltwater)
Duration:
28 days
Results:
The study found that both the 20 mg/kg and 40 mg/kg doses of cordycepin significantly increased the swimming time of weight-loaded mice. Taurine also exhibited a positive effect. Furthermore, cordycepin was found to reduce levels of lactic acid, which is associated with fatigue and muscle soreness following intense physical activity. Moreover, cordycepin increased the levels of energy metabolites (compounds involved in energy production) and antioxidants such as superoxide dismutase, glutathione, and NADP+. These findings suggest that cordycepin may alleviate fatigue by enhancing energy production, regulating energy metabolism, and increasing antioxidants.
Year:
2022
Link:
Study 3
Study type:
Rodent study
Purpose:
To investigate the anti-fatigue and anti-stress effects of Cordyceps sinensis mycelia in mice.
Dose:
150 and 300 mg/kg/day Cordyceps sinensis via a stomach tube
Results:
The swimming endurance of mice improved significantly when they were administered with Cordyceps sinensis mycelia at doses of 150 and 300 mg/kg per day. This resulted in a prolonged swimming time from 75 to 90 minutes and reduced fatigue. It effectively suppressed weight changes in the adrenal gland, spleen, thymus, and thyroid, potentially indicating a mitigating effect on stress-induced changes in these tissues. Furthermore, the hot water fraction inhibited an increase in total cholesterol and a decrease in alkaline phosphatase, which are biochemical indicators of the stress that arises when individuals are unable to move or change positions freely, resulting in feelings of discomfort, tension, and occasionally anxiety or frustration (known as immobilisation stress).
Year:
2003
Link:
Study 4
Study type:
Rodent study
Purpose:
To evaluate the anti-fatigue effects of Cordyceps militaris on mice.
Dose:
First group: 5, 10, and 20 g/kg of Cordyceps militaris combined with extruded products of cereal grains. Extruded products of cereal grains are food items made by processing cereal grains, like wheat or corn, through a machine that applies heat and pressure.
Second group: 5, 10, and 20 g/kg of the extruded product of cereal grains alone.
Third group: control (distilled water)
Duration:
30 days
Results:
Both the extruded product of cereal grains alone and in combination with Cordyceps militaris were able to prevent exercise-induced fatigue in mice. However, the extruded product of cereal grains with Cordyceps militaris were superior in relieving fatigue compared to the extruded product of cereal grains alone.
The study showed that supplementing mice with Cordyceps militaris reduced fatigue, improved their endurance during exercise, lowered muscle damage and stress, and decreased oxidative stress. Oxidative stress happens when there are too many harmful molecules called free radicals in the body, which can contribute to fatigue. Cordyceps helps by reducing these free radicals, protecting the cells from damage, and leading to higher energy levels and less fatigue.
Year:
2017
Link:
Study 1
Study type:
Rodent study
Purpose:
To evaluate the effect of Cordyceps cicadae extract in mice with testicular damage.
Dose:
50, 100, and 400 mg/kg body weight/day of Cordyceps cicadae extract rich in nucleosides
Duration:
7 days
Results:
Cordyceps treatment significantly restored testes weight loss, improved the testes index, and enhanced sperm parameters such as counts, viability, and motility in mice with testicular damage. Additionally, cordyceps treatment resulted in a significant increase in levels of testosterone and follicle-stimulating hormone. Elevated levels of serum testosterone indicate improved male hormonal balance, potentially leading to enhanced sexual function, while increased levels of follicle-stimulating hormone indicate that the reproductive organs are being properly stimulated, which is important for healthy reproductive function. Cordyceps also restored antioxidant activities in the testes and reduced inflammation. Improved antioxidant activity and reduced inflammation may protect the testes from damage and support the optimal functioning of sperm cells. Overall, these findings indicate that the treatment holds promise for improving male reproductive health.
Year:
2020
Link:
Study 2
Study type:
Rodent study
Purpose:
To explore the effects of cordycepin, a compound found in Cordyceps militaris, on testicular function in middle-aged rats.
Dose:
5, 10, 20 mg/kg of cordycepin or control
Duration:
4 months
Results:
Cordycepin improved sperm motility or movement, which is essential for successful fertilisation as sperm needs to reach and penetrate the egg. Specifically, a dosage of 20 mg/kg body weight was particularly effective in enhancing testicular function in middle-aged rats. Cordycepin administration also led to a slight increase in testosterone levels in middle-aged rats; however, this increase was not statistically significant. The findings suggest that cordycepin has the potential to restore normal testicular function and may serve as an effective pharmacologic agent to counteract the decline in testicular function associated with ageing.
Year:
2012
Link:
Study 3
Study type:
Rodent study
Purpose:
To investigate the protective effects of Cordyceps militaris extract in mice with reproductive damage induced by bisphenol A (BPA), a compound that can harm the testicles causing oxidative stress. Oxidative stress occurs when there are too many unstable molecules called free radicals in the body and not enough antioxidants to get rid of them, causing damage to organs and tissues.
Dose:
200, 400, 800 mg/kg body weight/day of Cordyceps militaris extract
Duration:
28 days
Results:
Cordyceps militaris protected against reproductive damage caused by BPA (bisphenol A). Mice exposed to 200 mg/kg BPA for 4 weeks experienced reproductive damage, but treatment with Cordyceps militaris reduced this damage. Cordyceps militaris significantly increased the levels of natural antioxidants in the testicles and reduced markers of oxidative stress.
Furthermore, Cordyceps militaris treatment significantly increased luteinizing hormone and testosterone levels in the blood, which can improve sperm count and motility. These findings demonstrate the potential of Cordyceps militaris as a natural substance to prevent reproductive damage caused by BPA.
Year:
2012
Link:
Study 4
Study type:
Cellular study (in-vitro)
Purpose:
To investigate the effects of Cordyceps sinensis on testosterone production in Leydig cells from male mice. Leydig cells are specialised cells found in the testes and are responsible for the synthesis and release of testosterone.
Dose:
3 mg/ml of Cordyceps sinensis
Results:
The study showed that Cordyceps sinensis at a concentration of 3 mg/mLl significantly increased the production of testosterone in Leydig cells. The researchers also investigated when the stimulation of testosterone production by Cordyceps sinensis was at its peak and found that it occurred between 2 to 3 hours after administration.
Year:
2001
Link:
Sexual Function
Study type:
Rodent study
Purpose:
To investigate the effects of Cordyceps militaris on sexual performance and erectile function in diabetic male rats. It is important to note that diabetes is strongly associated with sexual dysfunction in males.
Dose:
0.1, 0.5, and 1.0 g/kg/day of Cordyceps militaris or a single administration of 5 mg/kg of sildenafil (Viagra), or control
Duration:
3 weeks
Results:
The study revealed that daily administration of Cordyceps militaris at doses of 0.1 and 0.5 g/kg, as well as a single administration of Viagra, resulted in a significant increase in sperm count, sperm motility, testosterone levels, and overall improvement in sexual function. Additionally, the levels of penile nitric oxide synthase (NOS) and testicular superoxide dismutase (SOD) activities were significantly increased. Higher levels of penile nitric oxide synthase can promote better blood flow and enhance erectile function, while increased testicular superoxide dismutase activity can potentially preserve testicular health and function.
However, the 1 g/kg Cordyceps militaris treatment did not demonstrate statistical significance in the previously mentioned parameters, except for its effect on erectile activity, where a significant increase was observed in all treatment groups.
Overall, the results indicate that treatment with Cordyceps militaris at appropriate doses can improve erectile activity in diabetic rats. While both Cordyceps and Viagra treatments can enhance sexual function, it is important to note that Viagra has been associated with certain adverse effects. Therefore, Cordyceps treatment may offer a potential alternative or complementary approach for improving sexual function.
Year:
2020
Link:
Study 1
Study type:
Randomised clinical trial
Purpose:
To investigate whether Cordyceps sinensis can regulate the immune system in kidney transplant patients.
Dose:
3.0 g/day of Cordyceps sinensis (3 x 1.0 g capsule)
Participants:
182 transplant recipients with an average age of 38 years
Duration:
12 months
Results:
The group treated with Cordyceps sinensis reported having fewer complications after the transplant compared to the group that didn't take Cordyceps. Additionally, the group treated with Cordyceps exhibited higher levels of IL-10, a substance known for its anti-inflammatory properties. No serious side effects were observed.
Year:
2011
Link:
Study 1
Study type:
Rodent study
Purpose:
To evaluate the anti-diabetic activity of Cordyceps cicadae polysaccharide in diabetic rats.
Dose:
100, 200, 400 mg/kg body weight/day of Cordyceps cicadae polysaccharide or anti-diabetic medication (glibenclamide)
Duration:
30 days
Results:
In rats, Cordyceps cicadae polysaccharide had anti-hyperglycemic effects (meaning it lowered high blood sugar), anti-hyperlipidemic effects (meaning it lowered cholesterol and triglycerides in the blood), and antioxidant effects. The highest dose of 400 mg/kg led to a 45% reduction in blood sugar levels on day 10, with a peak reduction of 73% on the 20th day. This reduction was comparable to the effect observed in rats treated with anti-diabetic medication (glibenclamide), which resulted in a 75% reduction on the 20th day. Significant reductions in blood sugar levels were also observed at doses of 100 mg/kg and 200 mg/kg.
Cordyceps cicadae polysaccharide also increased beneficial substances in the body while decreasing harmful substances. Furthermore, researchers observed a decrease in urea, creatinine, ALT (Alanine aminotransferase), AST (Aspartate aminotransferase), and ALP (Alkaline phosphatase). A decrease in these substances signifies an improvement in kidney function and liver health.
Year:
2018
Link:
Shilajit Scientific Studies
The effects of shilajit have been extensively investigated through numerous clinical trials and rodent studies. We have summarised some of the most interesting scientific studies related to testosterone and sperm, performance enhancement, antioxidants, bone fractures, liver health, and skin health.
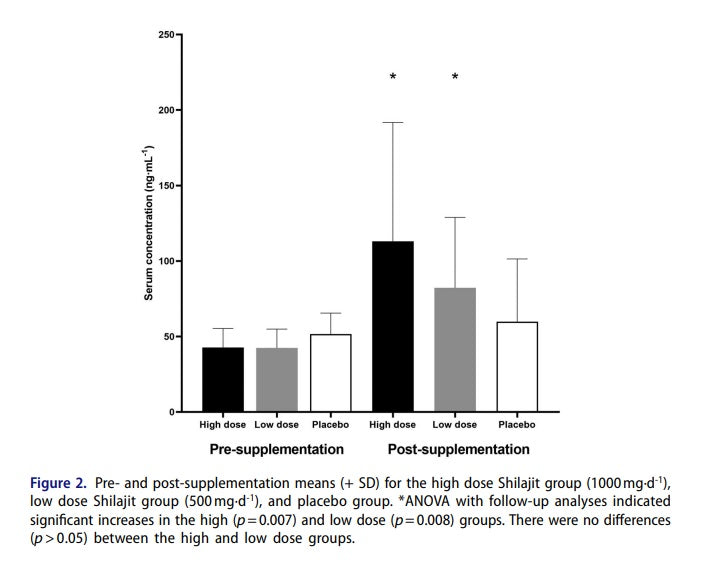
Study 1
Study type:
Randomised, double-blind, placebo-controlled clinical trial
Purpose:
To investigate the effects of shilajit on blood “pro-collagen type 1 alpha 1”, a fundamental molecule in collagen production, in recreationally trained men.
Dose:
500 mg/day of shilajit (low-dose) or 1000 mg/day of shilajit (high-dose) or placebo
Participants:
35 recreationally trained men with an average age of 21 years
Duration:
8 weeks
Results:
The study found an association between 8 weeks of shilajit supplementation (both high and low doses) and significant increases in “pro-collagen type 1 alpha 1” blood levels, compared to pre-supplementation levels. This increase in levels of pro-collagen type 1 alpha 1 indicates that collagen production also increased, which can contribute to improved joint and tissue health. This can potentially enhance overall physical activity and structural support in the body.
A higher number of participants taking 1000 mg/day of shilajit experienced a significant increase in blood levels of pro-collagen type 1 alpha 1 (75%) compared to the placebo group (30%). No differences between the high and low dose shilajit groups (69%) or between the low dose Shilajit group and placebo group were observed.
Year:
2022
Link:
Study 2
Study type:
Randomised, double-blind, placebo-controlled clinical trial
Purpose:
To evaluate the effects of shilajit supplementation on muscular strength and blood hydroxyproline levels. Hydroxyproline is an amino acid that is a key component of collagen (a protein found in bones, cartilage, skin and muscles). Blood levels of hydroxyproline are used as an indicator of collagen breakdown from muscle and connective tissue following high-intensity exercise. This breakdown isn't necessarily a negative response, as when muscles experience stress (like from exercise), they can adapt and become stronger. However, consistently elevated hydroxyproline levels due to frequent, intense exercise without adequate recovery might suggest excessive damage and collagen breakdown. Over time, without proper rest and recovery, this can lead to overtraining or an increased risk of injury and weaker skin, joints, and bones.
Dose:
250 mg/day (low-dose) and 500 mg/day (high-dose) of shilajit or placebo
Additional intervention:
Leg extension exercise routine to induce muscle fatigue – a total of 100 leg extensions completed within a 5 minute period.
Participants:
63 recreationally-active men with an average age of 21 years
It is important to note that the subjects were divided into upper and lower 50th percentiles based on their pre-supplementation maximal voluntary isometric contraction (the strongest force a muscle can produce without moving) and blood hydroxyproline levels. The "upper 50th percentile" group would include individuals who had pre-supplementation levels of maximal voluntary isometric contraction and blood hydroxyproline higher than or equal to at least half of the others in the study. The "lower 50th percentile" group would include individuals who had pre-supplementation levels of maximal voluntary isometric contraction and blood hydroxyproline lower than at least half of the others in the study. This division was carried out in order to gain insights into the potential benefits of Shilajit supplementation in individuals with higher initial strength and hydroxyproline levels.
Duration:
8 weeks (56 days)
Results:
In simple terms, participants who took 500 mg/day of shilajit had a better ability to maintain their muscle strength after exercise compared to those who took a placebo or a smaller dose.
In more technical terms, compared to the placebo and low-dose groups, participants taking the high dose of shilajit (500 mg/day) had a significantly lower “percent decline” in maximal voluntary isometric contraction (the strongest force a muscle can produce without moving) after exercise. A lower percent decline in maximal voluntary isometric contraction means that the muscles were less affected by fatigue or exertion, which can be a positive indicator of muscle endurance or recovery. These positive effects were particularly evident in participants who started the study with the highest levels of strength and hydroxyproline (upper 50th percentile group).
The study also found an association between the higher dose of shilajit supplementation (500 mg/day) and significantly lower hydroxyproline levels in participants in the upper 50th percentile group, compared to the low-dose and placebo groups (after statistical adjustment). This decline in hydroxyproline levels potentially indicated a reduction in collagen breakdown from connective tissues, but not muscle (muscle damage often results from eccentric muscle actions, whilst this study involved concentric muscle actions), suggesting a potential benefit for connective tissue health (tendon and ligament health) and a reduced risk of injuries. It is important to note that consistently elevated hydroxyproline levels might suggest excessive tissue damage and collagen breakdown. Over time, without proper rest and recovery, this can lead to weaker joints and tendons.
Overall, the findings of the study suggest that individuals who are already physically fit and have higher levels of baseline hydroxyproline may benefit more from shilajit supplementation in terms of its potential effect on exercise performance.
Year:
2019
Link:
Study 3
Study type:
Clinical trial (uncontrolled)
Purpose:
To investigate the effects of shilajit extract on muscle adaptation in overweight adults. Muscle adaptation refers to the changes that occur in muscles, such as increased strength and growth, in response to exercise or other environmental factors. Overweight individuals are at risk of reduced muscle mass, impaired muscle function, and difficulties in engaging in physical activity.
Dose:
500 mg/day of shilajit (2 x 250 mg capsules)
Additional intervention:
Treadmill exercise for the last 4 weeks of the study
Participants:
16 overweight adults aged 21-70 years
Duration:
12 weeks
Results:
The study found an association between oral shilajit supplementation and improved muscle adaptation through the activation of extracellular matrix-related genes. These extracellular matrix-related genes help to improve muscle elasticity, repair, and regeneration, and help in transfer mechanical forces during muscle contraction. In simpler terms, the study found that taking shilajit supplements might help muscles get better at handling exercise and become stronger by activating certain genes related to the muscle's structure. Improved muscle adaptation is beneficial because it enhances physical performance, reduces the risk of injuries, improves metabolic health, aids in weight management, and contributes to overall well-being and longevity.
The researchers also observed that shilajit supplementation alongside 4 weeks of treadmill exercise further increased the expression of these beneficial genes, further enhancing the positive effects on muscle strength and health. No negative effects of shilajit supplementation were reported.
Year:
2016
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled clinical trial
Purpose:
To evaluate the effects of processed shilajit on testosterone levels in healthy volunteers.
Dose:
500 mg/day of purified shilajit (2 x 250 mg capsules) or placebo
Participants:
75 healthy male volunteers aged 45-55 years
Duration:
90 days
Results:
The researchers observed significant increases in the total testosterone levels (20.45%) and free testosterone levels (19.14%) after 90 days of shilajit supplementation compared to the levels at the start of the study. The placebo group, on the other hand, experienced significant decreases in testosterone levels in the placebo group.
Moreover, the study found an association between shilajit supplementation and a 31.35% increase in dehydroepiandrosterone (DHEA) levels, which is a precursor to testosterone. Elevated DHEA levels may help to increase levels of male sex hormones, including testosterone.
Regarding changes in the levels of luteinizing hormone and follicle-stimulating hormone (hormones that play important roles in the regulation of the reproductive system), the shilajit-treated group and placebo group did not differ significantly.
Overall, the study found a correlation between shilajit supplementation and increases in total and free testosterone levels, the maintenance of luteinizing and follicle-stimulating hormones, and increases in the testosterone precursor DHEA.
Year:
2016
Link:
Study 2
Study type:
Clinical trial (uncontrolled)
Purpose:
To evaluate the effects of shilajit on sperm production in male patients with low sperm count (oligospermic).
Dose:
200 mg/day of processed shilajit (2 x 100 mg capsules)
Participants:
28 male patients with low sperm count aged 30-45 years
Duration:
90 days
Results:
The researchers observed a significant increase in total sperm count (61.4%), sperm movement (12.4%-17.4%) and normal sperm count (18.9%) in male patients following shilajit supplementation.
Higher levels of testosterone (23.5%) and follicle-stimulating hormone (9.4%) were also observed, indicating a potential positive effect on male reproductive health.
The study also found an association between shilajit supplementation and a decrease in MDA (malondialdehyde) level in semen. Lower levels of MDA in semen are generally seen as beneficial for fertility and reproductive function. MDA is a marker of oxidative stress, and higher levels of oxidative stress can harm cells and have negative effects on various bodily functions, including reproductive health.
No negative reactions were reported by any patients during the treatment period. The study suggests that shilajit treatment is safe and may have beneficial effects on sperm production and overall male reproductive function.
Year:
2010
Link:
Study 3
Study type:
Case studies
Purpose:
To evaluate the effects of shilajit on sperm production in male patients with low sperm count (oligospermic).
Participants:
4 patients with low sperm count (oligospermic) after 3 months of 200 mg daily oral supplementation of processed shilajit (2 x 100 mg capsules)
Results:
Case 1: A 37-year-old man with infertility issues showed improvement in sperm count and testosterone levels after 3 months of treatment with processed shilajit.
Case 2: A 30-year-old farmer with infertility issues also showed improvement in sperm count and testosterone levels after 3 months of treatment with processed shilajit.
Case 3: A 36-year-old businessman with anxiety and loss of strength experienced improvement in sexual activity and sperm count after 3 months of treatment with processed shilajit.
Case 4: A 32-year-old IT professional with infertility issues showed significant improvement in sperm count, motility, and testosterone levels after 3 months of treatment with processed shilajit.
All cases mentioned that their wives became pregnant after the 3-month treatment with shilajit.
The findings suggest that shilajit may be effective in improving sperm count, sperm motility, and testosterone levels in men with infertility issues.
Year:
2010
Link:
Study 4
Study type:
Rodent study
Purpose:
To investigate the effects of shilajit on infertility induced by cadmium in male mice. Cadmium is a toxic heavy metal associated with reduced sperm quality, decreased sperm motility, and lower sperm count.
Dose:
50, 100, and 200 mg/kg/day body weight of shilajit or control
Duration:
70 days (35 days of shilajit treatment)
Results:
The study found that all three doses of shilajit were effective at improving the reproductive health of male mice that suffered from infertility. Shilajit treatment resulted in positive changes, such as increased weight of reproductive organs, increased daily sperm production, higher testosterone levels, higher sperm concentration, improved sperm movement and restoration of sperm cell formation.
Shilajit also led to higher concentrations of sialic acid (a type of sugar) in the epididymis (a tube behind testicles that play a role in sperm transport, maturation, and storage) and higher concentrations of fructose in seminal vesicles (glands that produce fluid for semen), both of which are essential components of semen.
Moreover, shilajit enhanced libido, improved fertility and increased the number of offspring produced by female mice that mated with shilajit-treated male mice.
The findings indicate the potential of shilajit in improving semen quality and reproductive function.
Year:
2018
Link:
Study 5
Study type:
Rodent study
Purpose:
To evaluate the effects of shilajit on spermatogenesis (the process of sperm cell formation in the testes) and ovogenesis (the process of egg cell formation in the ovaries) in male and female rats.
Dose:
Male rats: 25, 50 and 100 mg/kg/day of shilajit or control
Female rats: 25 and 100 mg/kg/day of shilajit or control
Duration:
6 weeks
Results:
The study found that both 50 mg/kg and 100 mg/kg of shilajit treatment led to a significant increase in the number of sperm in the testes and epididymides (a coiled tube located behind each testicle which stores and transports sperm) of male rats.
After female rats were treated with 100 mg/kg of shilajit, there was a significant increase in the number of rats in the estrus stage. The estrus stage is a specific phase in the reproductive cycle of female mammals during which they are sexually receptive and capable of mating or copulation.
The results suggest that shilajit may have beneficial effects on both male and female fertility.
Year:
2006
Link:
Study 1
Study type:
Prospective, randomised, double-blind, placebo-controlled clinical trial
Purpose:
To investigate the effect of shilajit extract on bone mineral density (an essential indicator of bone strength and density) in postmenopausal women with osteopenia, a condition characterised by lower than normal bone density, but not as severe as osteoporosis.
Method of evaluation:
Bone mineral density was measured using imaging technology that uses X-rays to assess bone density.
Dose:
250 mg/day and 500 mg/day of shilajit extract or placebo
Participants:
59 postmenopausal women with osteopenia, aged 45-65 years
Duration:
48 weeks
Results:
The study found an association between daily supplementation with shilajit and a significant increase in bone mineral density compared to the placebo group. An increase in bone mineral density indicates stronger and denser bones as they contain more essential minerals like calcium and phosphorus, reducing the risk of fractures and conditions like osteoporosis. This improvement was more significant in participants taking a higher dose of 500 mg/day compared to those taking 250 mg/day. Additionally, the study also found an association between shilajit supplementation and significant reductions in bone turnover, which indicates a potential increase in or maintenance of bone density, though its impact on overall bone strength requires further investigation.
The researchers observed significant reductions in the levels of C-reactive protein, a marker of chronic inflammation associated with ageing, low bone mineral density, and fracture risk, at week 48 in both groups of women supplemented with shilajit, compared to the placebo group. There was also a significant reduction in malondialdehyde (MDA), an indicator of oxidative stress, along with a significant increase in glutathione, the body's primary antioxidant, at all timepoints in the shilajit supplemented groups, compared to the placebo group.
Overall, shilajit extract may be beneficial in supporting bone health and reducing the risk of bone-related issues in postmenopausal women with osteopenia.
Year:
2022
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled clinical trial
Purpose:
To evaluate the effects of shilajit on the healing process of tibia fractures (shin bone fractures)
Dose:
1000 mg of shilajit (2 x 500 mg capsules plus 250 mL water, 1 hr before meals) or placebo
Participants:
160 males and females with shin bone fractures aged 18-60 years (62 patients completed the study)
Duration:
28 days of shilajit treatment (follow-up every 4 weeks after treatment period)
Results:
Participants taking the shilajit supplement reported significantly shorter average healing times of their fractured shin bone, leading to a quicker restoration of its normal strength and function.
Only two participants in the treatment group experienced mild to moderate symptoms (headache), but it did not impact their participation in the study.
Overall, the study suggests that shilajit supplementation after shin bone fracture surgery is safe and may be a promising option to speed up the healing process of fractures.
Year:
2020
Link:
Study 1
Study type:
Rodent study
Purpose:
To evaluate the effect of shilajit on the liver damage caused by non-alcoholic fatty liver disease in mice.
Dose:
150 and 250 mg of shilajit or 10 mg of pioglitazone (a prescription medication for type 2 diabetes) or controls
Duration:
2 weeks
Results:
Mice treated with shilajit and pioglitazone were found to have significantly improved levels of blood sugar, triglycerides, total cholesterol, liver enzymes associated with liver damage, and low-density lipoprotein (so-called “bad” cholesterol) compared to the control groups. High levels of low-density lipoprotein, “bad” cholesterol, may increase the risk of heart disease and stroke. The treated mice also experienced increased levels of high-density lipoprotein (“good” cholesterol), which is generally considered beneficial because it plays a vital role in removing excess cholesterol from the arteries and transporting it back to the liver for processing and excretion.
Moreover, the use of shilajit also restored the balance between antioxidants and oxidants (unstable molecules in the body that can cause cell and tissue damage), leading to a substantial improvement in the antioxidant system (the body’s natural defence against harmful molecules) in both the shilajit and pioglitazone groups.
The study suggests that both shilajit and pioglitazone hold promise as treatments for non-alcoholic fatty liver disease due to their antioxidant properties and potential ability to improve levels of lipids in the blood. Considering the known side effects of pioglitazone, shilajit may be a preferable option for helping to manage non-alcoholic fatty liver disease with the added benefit of supporting cardiovascular health.
Year:
2020
Link:
Study 1
Study type:
Randomised, placebo-controlled clinical trial
Purpose:
To investigate the effects of shilajit supplementation on the activity of genes and microperfusion (the flow of blood in tiny blood vessels) in the skin in healthy adult females.
Dose:
250 mg/day (low-dose) and 500 mg/day (high-dose) of standardised shilajit extract (2 x 125 mg or 250 capsules) or placebo
Participants:
45 female subjects aged 30-65 years
Duration:
14 weeks
Results:
The researchers observed a heightened reddish hue in the skin among participants taking the 500 mg daily dose of shilajit, indicating improved microperfusion, meaning that more oxygen and nutrients are being delivered to the skin cells, and waste products are being removed more efficiently, which is generally considered a positive sign of improved skin health and function.
The study also found an association between shilajit supplementation and the activation of specific genes involved in the movement of endothelial cells (the process in which the cells that line the interior surface of blood vessels migrate from one location to another within the blood vessel) and growth of blood vessels. Both movement of endothelial cells and blood vessel growth are known to play a vital role in wound healing, angiogenesis (the formation of new blood vessels), and inflammation.
In participants taking shilajit, researchers observed increased activation of genes related to the extracellular matrix (a complex network of molecules that provide structural support to cells and tissues). Activation of these genes is beneficial as it contributes to cellular repair and maintains tissue health, helping to ensure proper functioning of the body's organs and tissues. The extracellular matrix is also known to play an essential role in the development, maintenance, and regeneration of skeletal muscles.
These findings suggest that oral shilajit supplementation may help to improve blood vessel-related processes and blood flow in the skin.
Year:
2019
Link:
PRODUCTS CONTAINING SHILAJIT
PrimaVie® Shilajit 400mg

Berberine HCl Scientific Studies
Numerous clinical trials have investigated the effects of berberine in humans. We have summarised some of the most important studies related to cardiovascular health, gastrointestinal health, metabolic health, blood sugar and insulin sensitivity
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To evaluate berberine in the treatment of type 2 diabetic patients with abnormal levels of lipids (fats) in the blood.
Dose:
1 g of berberine (2 x 0.5 g tablets) or placebo
Participants:
116 male and female patients with type 2 diabetes, aged 51 on average
Duration:
3-month treatment period
Results:
Fasting blood sugar decreased from 7.0 to 5.6 mM/litre after long-term Berberine supplementation, compared to a smaller reduction of 6.8 to 6.4 mM/litre after the placebo.
Levels of triglycerides, total cholesterol, and "bad" cholesterol decreased following long-term berberine supplementation, in contrast to the increases or minor reductions observed after the placebo.
Participants taking Berberine also experienced a greater reduction in body weight and BMI compared to the placebo group.
Year:
2008
Link:
Study 2
Study type:
Clinical trial
Purpose:
To compare the effects of berberine and metformin, a commonly prescribed type 2 diabetes medication, in individuals with diabetes.
Dose:
Study A: 1500mg of berberine or 1500mg metformin per day
Study B (uncontrolled): 1500mg of berberine per day, in addition to the participants’ existing diabetes medication.
Participants:
Study A: 36 male and female newly diagnosed patients for type 2 diabetes
Study B: 48 male and female type 2 diabetes patients with existing anti-diabetic treatment
Duration:
13 weeks
Results:
Researchers observed in diabetics taking berberine improved blood work, including key diabetes metrics like fasting blood sugar, similar to the effects associated with the diabetes medication metformin. Additionally, when combined with existing diabetes treatments, berberine was associated with improvements in blood sugar control, insulin sensitivity, and insulin secretion. 34.5% of participants reported temporary gastrointestinal side effects, which mostly resolved after reducing the berberine dosage to 900 mg per day.
Year:
2008
Link:
Study 3
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To evaluate the effect of berberine on blood sugar levels and cholesterol levels in prediabetics
Dose:
376 mg/day of berberine (2 x 188 mg of berberine tablets, taken before lunch and dinner) or placebo
Participants:
49 male and female overweight subjects with an average age of 60 years
Duration:
2 months
Results:
Participants who took berberine experienced better blood sugar levels and overall health improvements, including a lower risk of cardiovascular problems and better sugar control, compared to those who took a placebo. No side effects were reported.
Year:
2023
Link:
Study 1
Purpose:
To examine Berberine's effects in patients with chronic heart failure, a medical condition where the heart is unable to pump blood effectively, leading to inadequate circulation of blood throughout the body.
Dose:
1.2-2.0 g/day of berberine (4 x 0.3-0.5 g tablets) or placebo
Participants:
156 male and female patients with chronic congestive heart failure, with an average age of 64 years
Duration:
8 weeks
Results:
Participants with heart-failure who took berberine experienced significantly greater improvements in heart function and exercise capacity compared to the control group. They also reported less shortness of breath, less fatigue, and fewer abnormal heartbeats, and long-term follow-up revealed fewer deaths due to sudden cardiac death and congestive heart failure.
Year:
2003
Link:
Study 2
Study type:
Multicenter, double-blind, randomised, placebo-controlled, clinical trial
Purpose:
To evaluate the effects of berberine in patients with low cardiovascular risk.
Dose:
1000 mg/day of berberine (2 x 500 mg capsules, taken at lunch and dinner) or placebo
Participants:
144 male and female patients with an average age of 53 years (137 completed the study)
Duration:
6 months treatment period
Results:
Participants taking berberine experienced improved cholesterol levels, with decreases in total and bad cholesterol and increases in good cholesterol, unlike those in the placebo group who experienced no significant changes. Following a 2-month break from berberine, participants' cholesterol levels worsened, but improved again once berberine was reintroduced.
Year:
2013
Link:
Study 3
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To investigate the cholesterol-lowering effects of berberine.
Dose:
1 g/day of berberine or placebo
Participants:
91 man and women with elevated cholesterol levels
Duration:
3 months
Results:
After 3 months of berberine treatment, significant health improvements were observed: there was an 18% decrease in cholesterol, a 28% reduction in triglyceride levels, and a 20% reduction in bad cholesterol (LDL), with no similar observations in the placebo group. Further analysis focusing on individuals not taking other medications or special diets revealed even more pronounced effects of berberine, including a 29% drop in cholesterol, a 35% decrease in triglycerides, and a 25% reduction in bad cholesterol, while good cholesterol (HDL) remained unchanged. Berberine was well-tolerated, with the exception of one individual experiencing mild constipation, which was resolved by adjusting the dosage.
Year:
2004
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled clinical trial
Purpose:
To evaluate the effect of berberine supplementation on metabolic syndrome, which encompasses a range of health problems such as high blood pressure, high blood sugar, excess waist fat, and abnormal cholesterol or triglyceride levels. These issues collectively increase the risk of heart disease, stroke, and type 2 diabetes.
Dose:
1500 mg/day of berberine or placebo
Participants:
24 male and female patients with metabolic syndrome, aged 30-40
Duration:
3 months
Results:
Participants who took berberine experienced a 36% improvement in the symptoms of metabolic syndrome. This included significant improvements in waist circumference (a measure of abdominal obesity), systolic blood pressure, triglycerides (fat in the blood), blood sugar levels, total insulin secretion, and an increase in insulin sensitivity (how well the body uses insulin to control blood sugar).
Year:
2013
Link:
Study 1
Study type:
Randomised, double-blind, placebo-controlled trial
Purpose:
To investigate whether berberine hydrochloride effectively relieves diarrhoea-predominant irritable bowel syndrome.
Dose:
400mg of a berberine hydrochloride supplement or placebo (vitamin C)
Participants:
196 male and female patients aged 18-65 with irritable bowel syndrome
Duration:
14 weeks (8-week treatment period)
Results:
Participant questionnaires revealed that patients treated with berberine experienced significant improvements in symptoms of irritable bowel syndrome, including greater reductions in diarrhoea frequency, abdominal pain, and urgency to defecate over an 8-week period, compared to the control group.
Year:
2015
Link:
